Lewis Structure Guide
This is a super important lewis structure guide by which you can draw any lewis structure.
I’ve also provided different periodic table charts to avoid your headache.
Also, there is a SURPRISE gift for you at the end of this article.
So let’s dive right into it.
Table of contents:
- How to draw lewis structures (Steps)
- Periodic table with valence electrons
- Periodic table with electronegativity values
- More Lewis structures
- Your SURPRISE gift
How to draw lewis structures (6 Steps)
Any lewis structures can be drawn by using 6 simple steps.
Step #1: Calculate the total number of valence electrons
In the very first step, you have to calculate the total number of valence electrons of a given molecule. (Valence electrons are the electrons that are present in the outermost orbit of an atom).
Step #2: Select the center atom (H is always outside)
While selecting the center atom, always put the least electronegative atom at the center. But if the molecule contains hydrogen, then always put hydrogen outside.
Step #3: Connect each atoms by putting an electron pair between them
In this step, connect each bonding atom by simply putting 2 electrons (i.e an electron pair) between them. This electron pair between the atoms represents the chemical bond.
Step #4: Make the outer atoms stable
Complete the octet (or duplet on hydrogen) on outside atoms. If the valence electrons are left, then put the valence electrons pair on the central atom.
Step #5: Check the octet on the central atom. If it does not have octet, then shift the lone pair to form a double bond or triple bond
In simple words, check whether the central atom is surrounded by 8 electrons or not. If it is not having 8 electrons, then shift the lone pair from the outer atoms. This shifted lone pair will form a chemical bond.
Step #6: Final step – Check the stability of lewis structure by calculating the formal charge on each atom
Now you have to calculate the formal charge on each atom. The formal charge on each atom should be zero. If the formal charge is not zero, then it should be minimized. This can be done by shifting the lone pair from negatively charged atom to the positively charged atom.
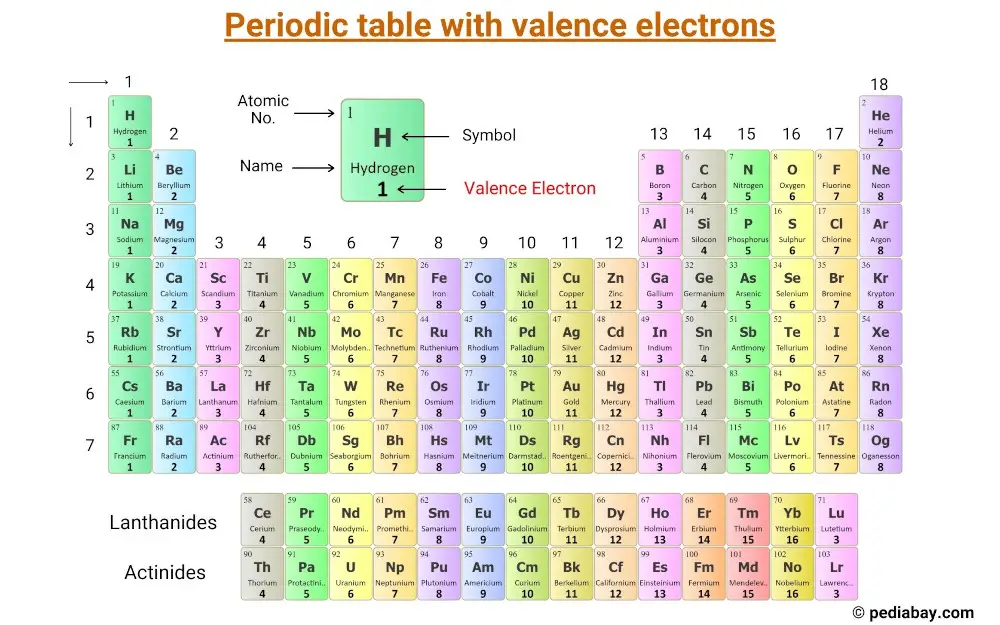
Periodic table with valence electrons
As mentioned in Step #1, you have to calculate the total number of valence electrons. This periodic table with valence electrons will help you find the valence electrons of each atom.
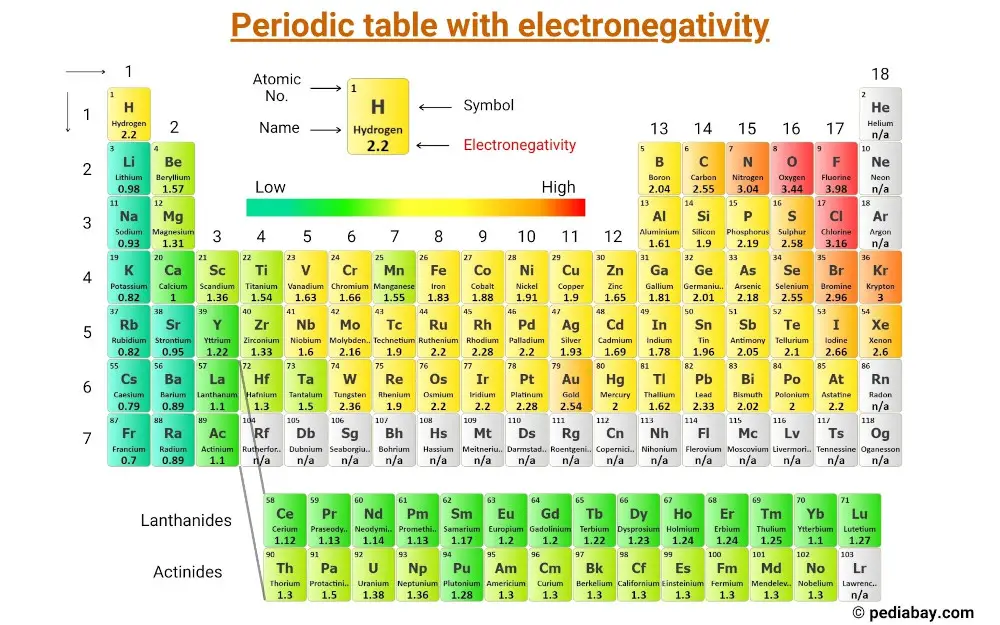
Periodic table with electronegativity values
For Step #2 (i.e selecting the central atom), you will need to see electronegativity values of the atoms. So this periodic table with electronegativity values will be helpful to you.
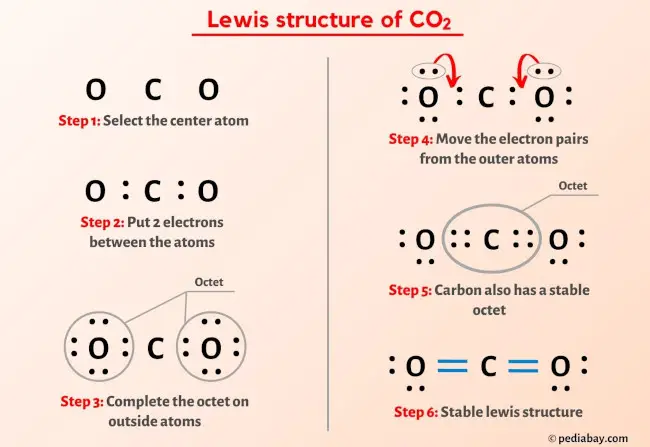
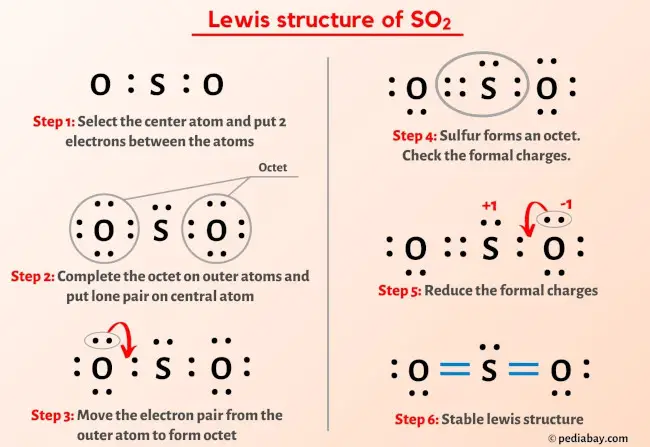
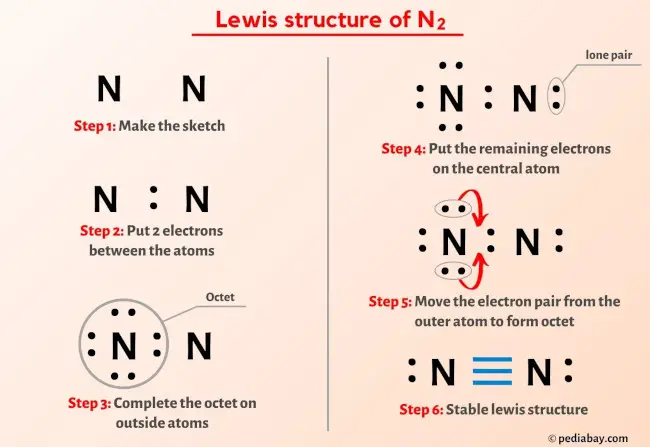
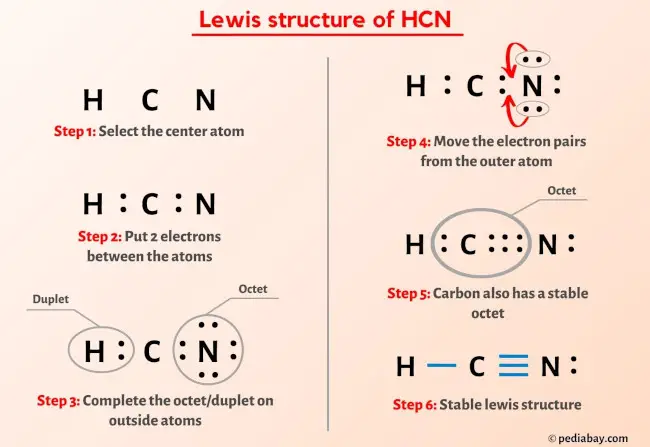
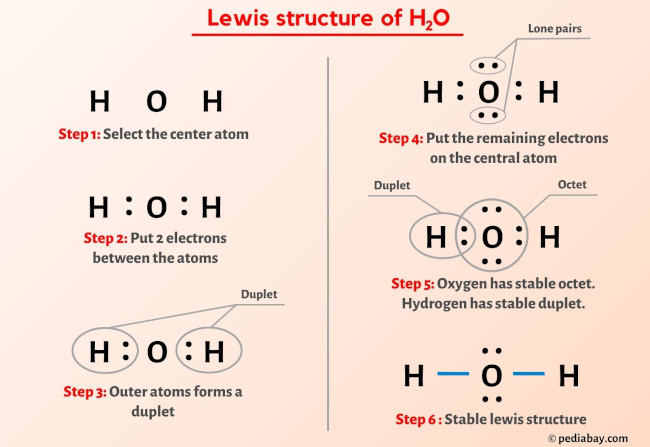
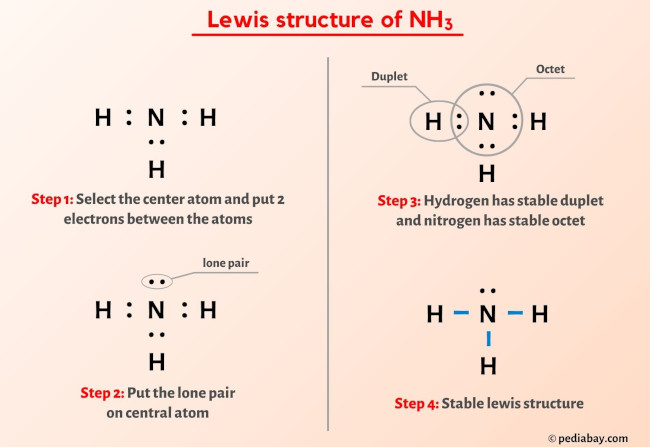
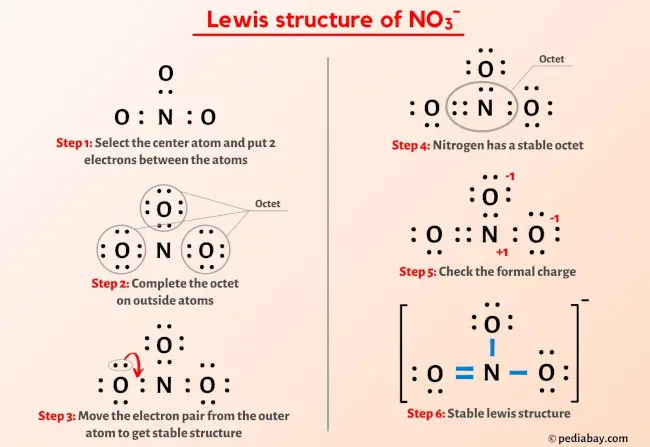
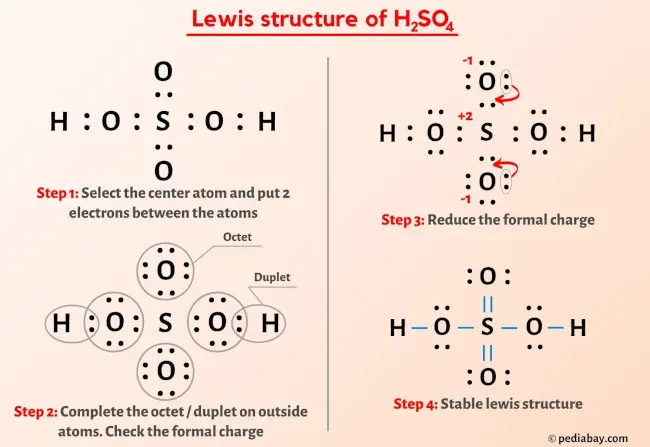
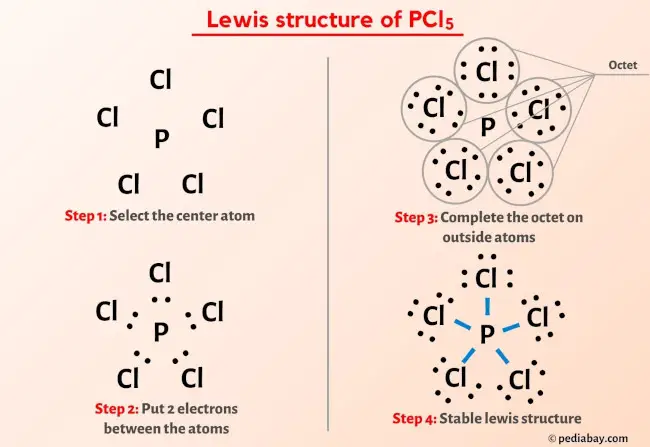
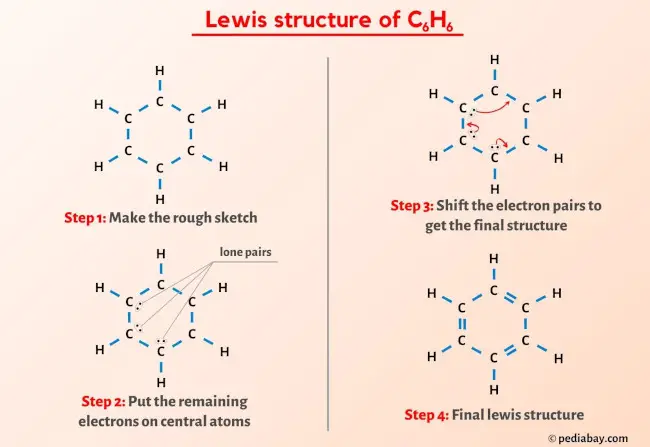
More Lewis structures
Here are a few Lewis structures for your practice that will make you an EXPERT. Open these individual lewis structures where I have explained how to draw these lewis structures using the 6 steps mentioned above.
Plus I’ve also used images and videos to explain each step. So you can refer to the below lewis structure for more practice.
Your SURPRISE Gift: Interactive Periodic Table
This is a complete interactive periodic table of elements with names, symbols, atomic mass, atomic number and much more information about each element.
Why is this Interactive Periodic Table Important?
- This Interactive periodic table contains almost every major data of all the 118 elements.
- There are different elements labeled on the periodic table to highlight that group of elements on the periodic table.
- Click on elements to see their rotating bohr diagrams.
- Click on search icons to see even more properties.
- Plus you can also download the HD image and PDF of this periodic table.
(Note: You must be on a laptop/desktop device for this interactive periodic table to function properly. Please visit this page from your laptop/desktop device).
Click on the above elements and search icon ( ![]() ) to see their interactive bohr diagrams and many other information like electron configuration, electrons arrangement, ionization energy, crystal structure, etc.
) to see their interactive bohr diagrams and many other information like electron configuration, electrons arrangement, ionization energy, crystal structure, etc.
Bookmark this Interactive Periodic Table
This Interactive periodic table is so important that it will be helpful to you in your entire academic career. So bookmark our website’s home page https://pediabay.com/. (I’ve also provided this Interactive periodic table on our website’s homepage for your easy access).
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.