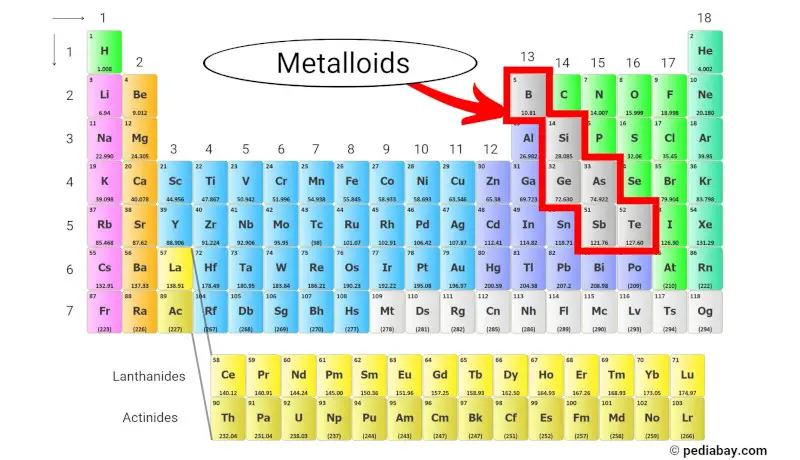
Metalloids are a group of elements found on the periodic table that have properties of both metals and nonmetals. They are also called semimetals.
They have some characteristics of metals, such as the ability to conduct electricity and heat, but also have properties of nonmetals, like being brittle and not shiny.
Let’s explore more about the metalloids.
Table of contents:
- What are metalloids?
- How many metalloids are on the periodic table?
- Semiconducting properties of metalloids
- Uses of metalloids
What are metalloids?
Metalloids are a group of elements in the periodic table that have intermediate properties between metals and nonmetals. They are also sometimes referred to as “semi-metals”.
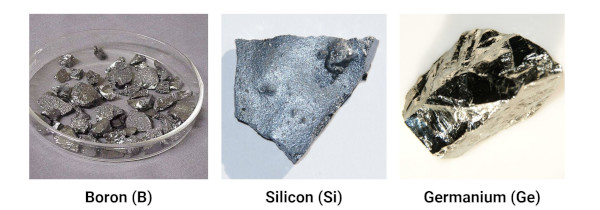
The metalloids include boron, silicon, germanium, arsenic, antimony, and tellurium.
Metalloids have characteristics of both metals and nonmetals. For example, they can conduct electricity to some extent, like metals, but they are not as good conductors as metals.
They also have some metallic luster and can be ductile and malleable, but not as much as pure metals. Additionally, they can form covalent bonds like nonmetals, but may also form ionic bonds with metals.
These elements are important in a variety of industries, including electronics, where they are used to create semiconductors and other electronic components. [1]
How many metalloids are on the periodic table?
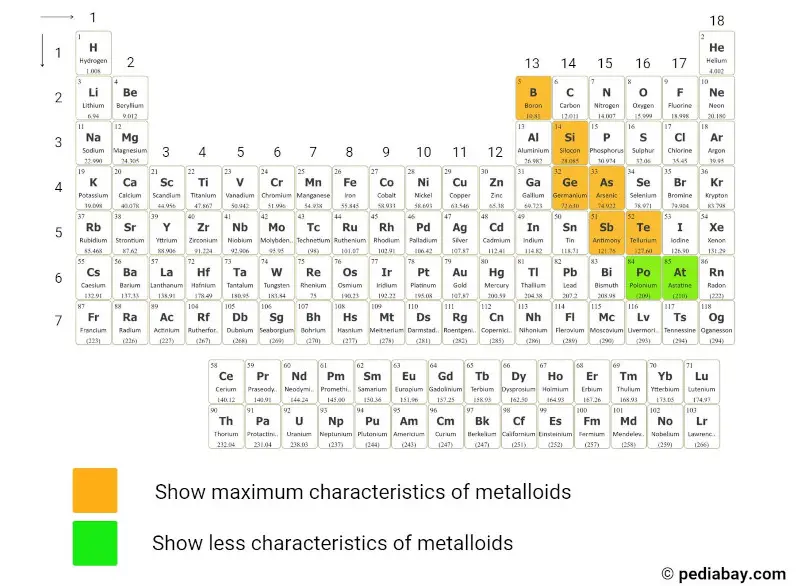
There are 6 commonly known metalloids on the periodic table.
They are;
- Boron
- Silicon
- Germanium
- Arsenic
- Antimony and
- Tellurium
But this is not completely true. Because polonium and astatine also show some characteristics of metalloids.
Are Polonium and Astatine considered metalloids?
The classification of Polonium and Astatine as metalloids is a topic of debate among researchers.
Some researchers argue that Polonium exhibits more metallic properties, while Astatine demonstrates more nonmetallic characteristics similar to the halogens. [2]
As a result, there is no universally accepted definition of metalloids. Different researchers may define them based on various criteria, including density, physical properties, or chemical properties.
Furthermore, due to their synthetic nature and extremely short half-lives, the classification of Polonium and Astatine is further complicated.
As it stands, the widely accepted list of metalloids or semimetals on the Periodic table includes six elements.
However, the ongoing research and evolving understanding of the properties of elements may lead to changes in the classification of Polonium and Astatine in the future.
Semiconducting properties of metalloids
Metalloids are elements that have semiconducting properties. This means that they possess a conductivity that is intermediate between metals and nonmetals.
Metalloids are not good conductors like metals, but their conductivity is higher than nonmetals.
The metalloids are called semiconductors because their energy band gap lies between that of conductors and insulators.
Let’s understand this with simple images.
Conductors:
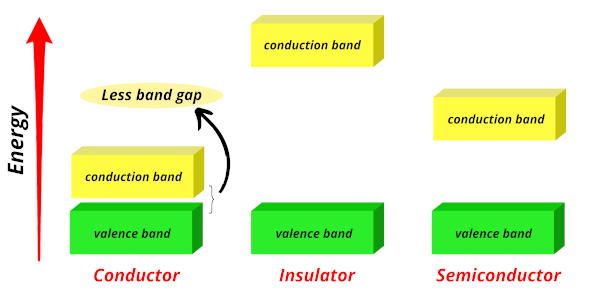
In conductors, the valence band and conduction band are very close to each other, allowing free electrons to easily jump from the valence band to the conduction band.
Insulators:
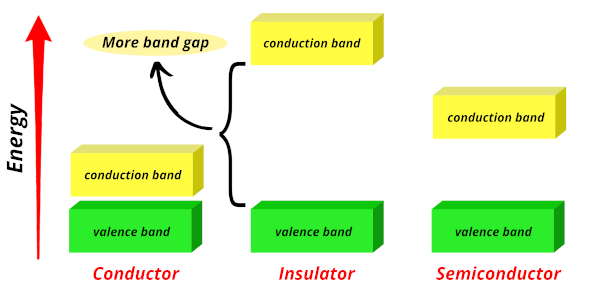
In insulators, the band gap is very large, requiring a large amount of energy for electrons to jump from the valence band to the conduction band.
Semiconductors:
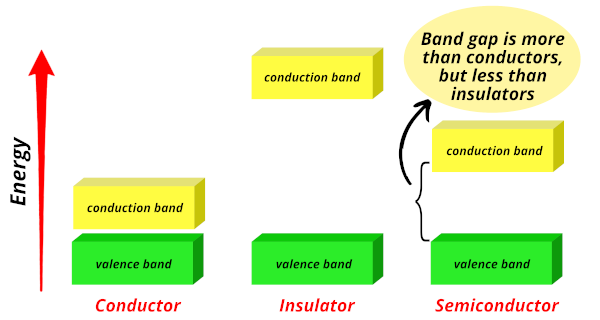
In contrast, the band gap of semiconductors is in between the two, meaning that electrons require less energy than that of insulators but more energy than that of conductors to jump from the valence band to the conduction band.
Hence the elements that have an energy band gap between that of conductors and insulators are classified as semiconductors.
Uses of metalloids
Metalloids have many different uses in a wide range of industries, from electronics to medicine to manufacturing. Here are a few uses of metalloids.
- Boron is used to make heat-resistant glass for things like ovenware and laboratory equipment. [3]
- Silicon is one of the most widely used metalloids in electronics. It is used to make computer chips, cell phones, and other electronic devices.
- Antimony is used to make certain types of batteries and bullets. [4]
- Germanium is used to make special lenses for infrared cameras that are used in night vision goggles. [5]
- Tellurium is used to make certain types of solar panels that can turn sunlight into electricity. [6]
- Metalloids are also used in the production of alloys, which are mixtures of metals. For example, arsenic is added to lead to make it harder, and boron is added to steel to make it stronger.
- Some metalloids, such as silicon and germanium, can be used to make semiconductors, which are used in electronic devices like computers and cell phones.
Summary
Metalloids are a group of elements with properties of both metals and nonmetals. They include boron, silicon, germanium, arsenic, antimony, and tellurium, with polonium and astatine also showing some characteristics of metalloids.
Metalloids have semiconducting properties, meaning they have intermediate conductivity between metals and nonmetals.
They are used in a variety of industries, including electronics, medicine, and manufacturing. Boron is used for heat-resistant glass, silicon for computer chips, arsenic for certain drugs, antimony for batteries and bullets, germanium for lenses in infrared cameras, and tellurium for solar panels. Some metalloids can also be used to make semiconductors for electronic devices.
External resources:
- Metalloid – Wikipedia. (n.d.). Metalloid – Wikipedia. https://en.wikipedia.org/wiki/Metalloid
- Foundation, C. (n.d.). CK12-Foundation. CK12-Foundation. https://flexbooks.ck12.org/cbook/ck-12-middle-school-physical-science-flexbook-2.0/section/4.5/primary/lesson/metalloids-ms-ps
- Metalloid | Definition, Elements, & Facts. (n.d.). Encyclopedia Britannica. https://www.britannica.com/science/metalloid
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.

