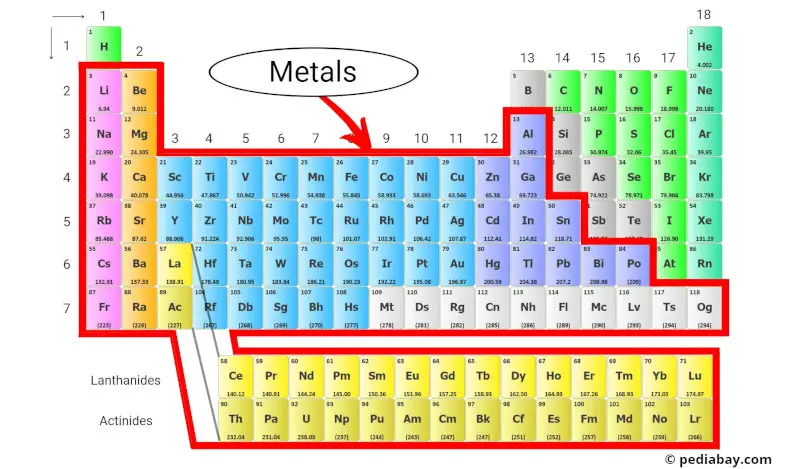
Metals of the periodic table are important elements that are found all around us, from the coins in our pockets to the wires that power our homes. In fact, most of the elements on the periodic table are metals!
They share some common properties, like being shiny, good conductors of heat and electricity, and malleable (which means they can be bent or shaped without breaking). [1]
Let’s explore more about the metals and the different types of metals present on the periodic table.
Table of contents:
- What exactly are the metals?
- Alkali metals
- Alkaline earth metals
- Transition metals
- Inner transition metals
- Rare earth metals
- Heavy metals
What exactly are the metals?
Metals are elements that tend to lose electrons and form cations (positively charged ions) when they undergo chemical reactions.
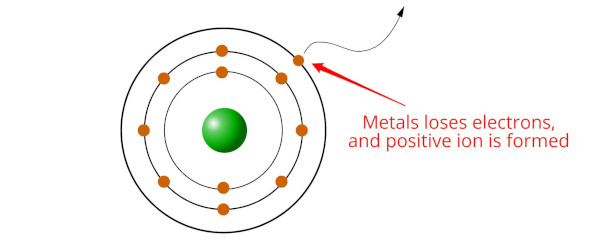
But do you know why this happens?
This is because metals have low ionization energies, meaning it requires relatively little energy to remove an electron from a metal atom. When a metal atom loses one or more electrons, it becomes a cation, which has a positive charge.
Metals tend to lose electrons in order to achieve a more stable electron configuration, typically by achieving a stable octet (i.e 8 electrons in the outermost shell).
Now let’s see the different types of metals classified on the periodic table.
Alkali metals
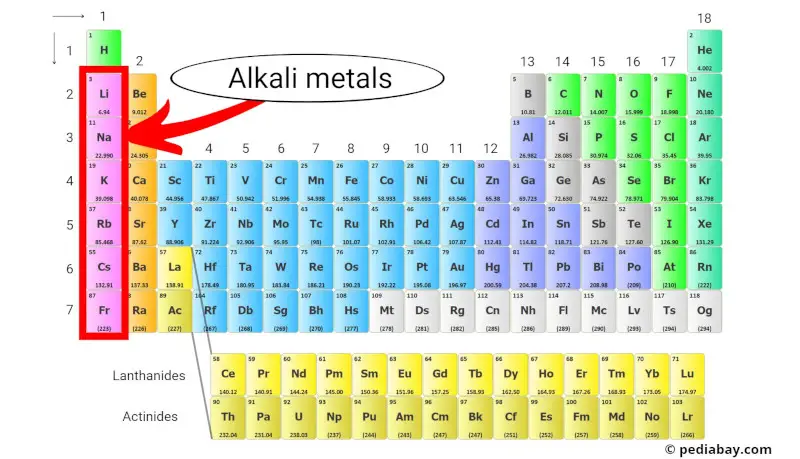
The alkali metals are a group of elements in the periodic table consisting of lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
They are located in Group 1 of the periodic table, which is also known as the “alkali metal group.”
Alkali metals are highly reactive due to their low ionization energies, which makes them more likely to lose their single valence electron to form a cation with a +1 charge. [2]
Read more about: Alkali metals of the periodic table.
Alkaline earth metals
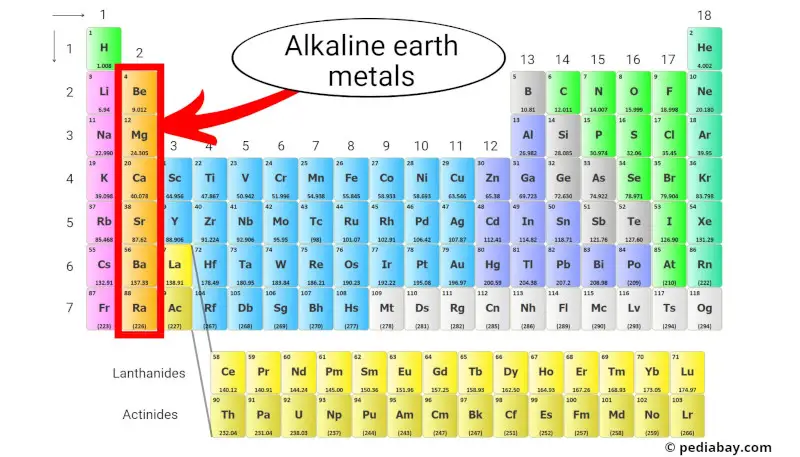
The alkaline earth metals are a group of elements in the periodic table consisting of beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
They are located in Group 2 of the periodic table, which is also known as the “alkaline earth metal group.”
Alkaline earth metals are less reactive than alkali metals, but they still have relatively low ionization energies and tend to form cations with a +2 charge. [3]
Read more about: Alkaline earth metals of the periodic table.
Transition metals
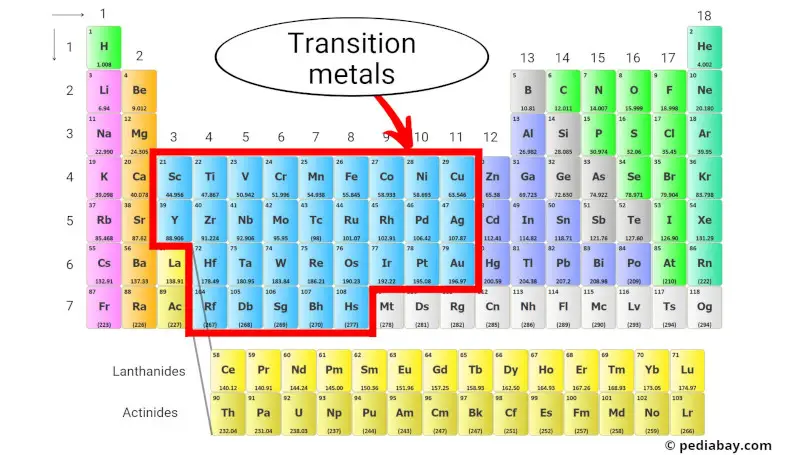
The transition metals are a group of metallic elements located in the center of the periodic table, occupying groups 3 through 11 (see the above image).
They are characterized by their ability to form colored compounds, multiple oxidation states, and their relatively high densities and melting points.
Transition metals are known for their chemical reactivity, with many of them being used as catalysts in chemical reactions. They are also used in a variety of applications, such as in the production of steel and other alloys, electronics, and batteries.
Read more about: Transition metals of the periodic table.
Inner transition metals
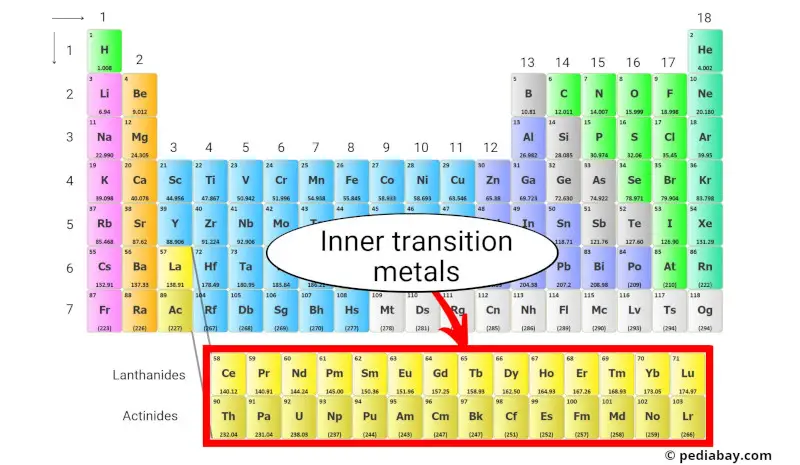
The inner-transition metals are a group of metallic elements that are located at the bottom of the periodic table, below the main body of the table.
They are divided into two subgroups:
- Lanthanides and
- Actinides.
The electronic configuration of inner-transition metals is unique, with electrons filling the f-orbitals of their atoms. This results in distinctive electronic and magnetic properties, making them highly useful in a variety of technological applications.
Read more about: Inner transition metals of the periodic table.
Rare earth metals
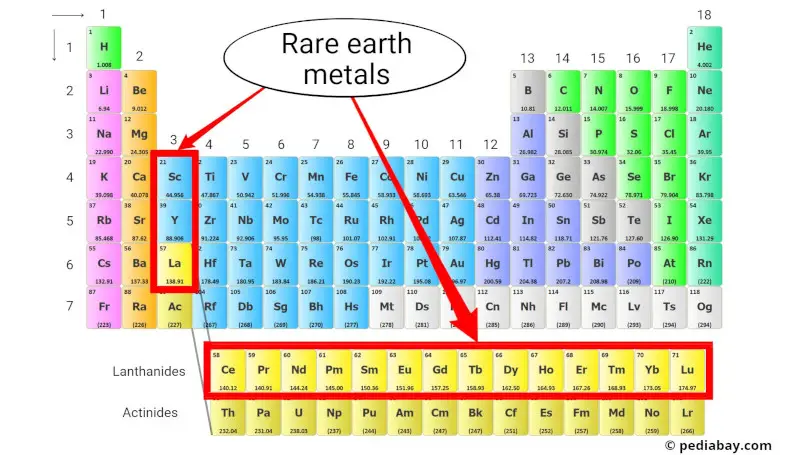
The rare earth metals, also known as the rare earth elements, are a group of metallic elements that include the lanthanide series (atomic numbers 57-71) and scandium (Sc) and yttrium (Y).
Despite their name, rare earth metals are not actually rare, but they are difficult and expensive to extract from ores due to their low concentrations and chemical similarities. [4]
Heavy metals
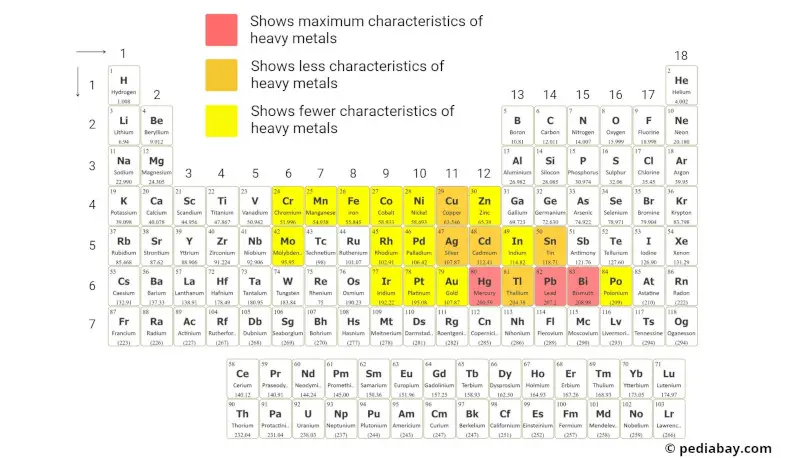
Heavy metals are a group of metallic elements that have a relatively high atomic mass and density.
Heavy metals are commonly identified by the criterion that they have a high density, typically greater than 5 g/cm³.
The above periodic table provides a visual representation of these elements based on this classification.
Read more about: Heavy metals of the periodic table.
Summary
Metals are elements that tend to lose electrons and form cations when they undergo chemical reactions. They have low ionization energies, making them more likely to lose electrons and become positively charged ions.
The periodic table contains several types of metals, including alkali metals, alkaline earth metals, transition metals, inner transition metals, rare earth metals, and heavy metals.
Alkali metals are highly reactive, while alkaline earth metals are less reactive. Transition metals are known for their ability to form colored compounds and multiple oxidation states, and inner transition metals have unique electronic and magnetic properties. Rare earth metals are difficult and expensive to extract, while heavy metals have high densities.
External resources:
- Boudreaux, K. A. (n.d.). The Parts of the Periodic Table. The Parts of the Periodic Table. http://www.angelo.edu/faculty/kboudrea/periodic/physical_metals.htm
- Metal – Wikipedia. (2021, March 25). Metal – Wikipedia. https://en.wikipedia.org/wiki/Metal
- Dye, J. L. (2015, March 13). The alkali metals: 200 years of surprises. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences, 373(2037), 20140174. https://doi.org/10.1098/rsta.2014.0174
- Information on Alkali Metals – Stanford Environmental Health & Safety. (n.d.). Information on Alkali Metals – Stanford Environmental Health & Safety. http://ehs.stanford.edu/reference/information-alkali-metals
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.