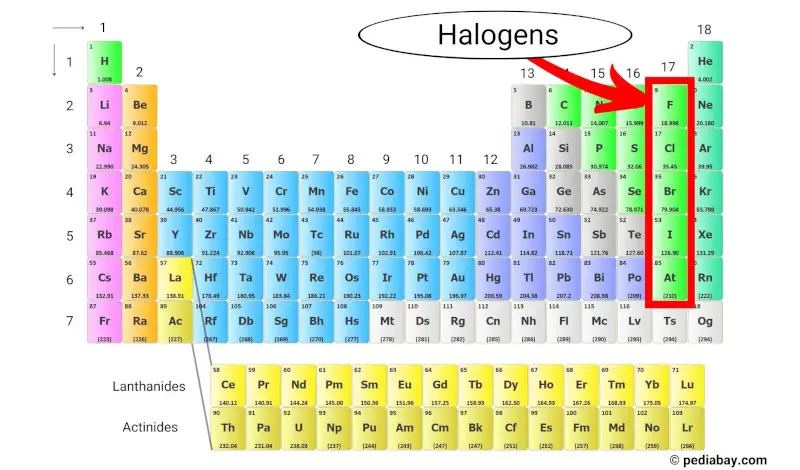
Halogens are a group of elements that are found in the group 17 (also known as halogen group) of the periodic table. The halogens include five different elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At).
Halogens are unique because they are the only group of elements that can form compounds with all other elements on the periodic table.
Let’s explore more about the halogens.
Table of contents:
- What are halogens?
- The most reactive halogen on the periodic table
- Reactivity of halogens
- Periodic trends of halogens
- Summary
What are halogens? And why are they called so?
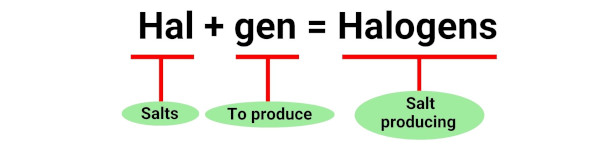
The term “halogen” is derived from the Greek words “halos” meaning salt and “genes” meaning producer. [1]
This is because halogens were first discovered by their ability to produce salts when they reacted with metals.
For example:
When chlorine (Cl2) reacts with sodium (Na), it forms sodium chloride (NaCl), which is common table salt:
2Na + Cl2 → 2NaCl
Similarly, when bromine (Br2) reacts with potassium (K), it forms potassium bromide (KBr):
2K + Br2 → 2KBr
These reactions are examples of halogens producing salts when they react with metals, which is where the name “halogen” comes from.
The most reactive halogen on the periodic table
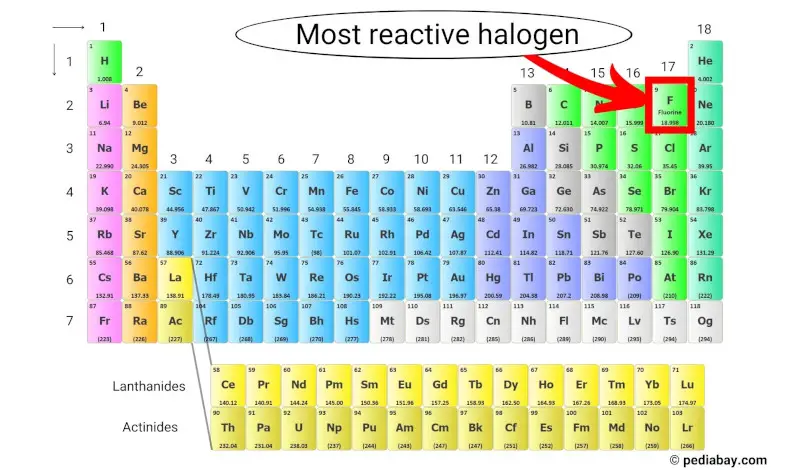
Fluorine is the most reactive halogen because it has the highest electronegativity of all the elements in the periodic table, meaning it has a strong attraction for electrons.
This makes it highly reactive and able to react with almost all other elements in the periodic table to form compounds. [2]
In simple terms, fluorine is like a magnet for electrons, and it will do anything it can to gain an electron and complete its outer shell.
It does this by reacting with other elements and stealing their electrons to form new compounds.
This is why fluorine is so reactive and why it is never found in its elemental form in nature, but instead always exists as part of a compound.
Because of its high reactivity, fluorine is also extremely dangerous and can be hazardous to work with.
It is often handled in specialized laboratories and is used in industrial processes to produce a wide range of chemicals and materials.
Reactivity of halogens
Halogens are highly reactive due to their higher electronegativities, which means they have a very high capacity to attract the electrons.
Also, halogens have 7 electrons in their outermost shell, and they only need 1 more electron to fill it up and achieve a stable electron configuration like the noble gases.
For example, you can see that the bohr diagram of fluorine has 7 electrons in its outermost shell.
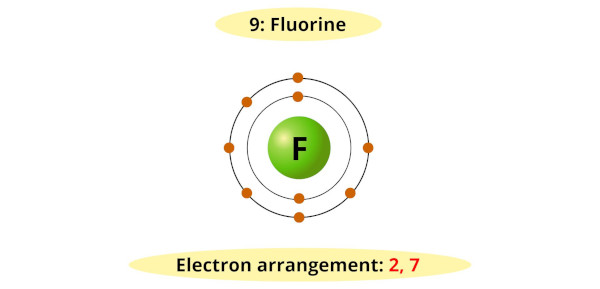
Now it requires 1 more electron to achieve a stable octet (7 + 1 = 8).
Halogens react with metals to form ionic compounds called metal halides, which can produce bright, colorful flames.
They also react with non-metals like hydrogen, sulfur, and nitrogen to form a range of covalent compounds. [3]
Hence the tendency of halogens to gain an electron and achieve a stable noble gas configuration makes them highly reactive and capable of forming compounds with other elements.
Periodic trends of halogens
As we move down the group, the halogen elements show changes in their physical properties as well as chemical properties.
Let’s see the trends of a few properties of halogens.
- Atomic size: As we move down the group, the atomic size of halogens increases. This is because each halogen has one more energy level than the one above it, which means that the outermost electrons are farther from the nucleus.
- Electronegativity: As we move down the group, the electronegativity of halogen elements decreases. This means that the halogens at the top of the group, such as fluorine and chlorine, are more electronegative and therefore more likely to attract electrons than the ones at the bottom, such as iodine and astatine.
- Melting points and boiling points: As we move down the group, the melting and boiling points of halogens increase. This is because the larger atoms have more electrons and are able to form stronger intermolecular forces. [4]
- Reactivity towards metals: As we move down the group, the reactivity of halogens towards metals decreases. This means that the halogens at the top of the group, such as fluorine and chlorine, are more likely to react with metals than the ones at the bottom, such as iodine and astatine.
Summary
Halogens are a group of five elements (fluorine, chlorine, bromine, iodine, and astatine) found in group 17 of the periodic table. They are unique in their ability to form compounds with all other elements.
Halogens are highly reactive due to their high electronegativities and tendency to gain an electron to achieve a stable noble gas configuration. Fluorine is the most reactive halogen due to its high electronegativity.
As we move down the group, halogens show changes in physical and chemical properties, such as increasing atomic size, decreasing electronegativity, and increasing melting and boiling points. The reactivity of halogens towards metals also decreases as we move down the group.
External resources:
- Halogen – Wikipedia. (2022, March 21). Halogen – Wikipedia. https://en.wikipedia.org/wiki/Halogen
- The Chemistry of the Halogens. (n.d.). The Chemistry of the Halogens. http://chemed.chem.purdue.edu/genchem/topicreview/bp/ch10/group7.php
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.