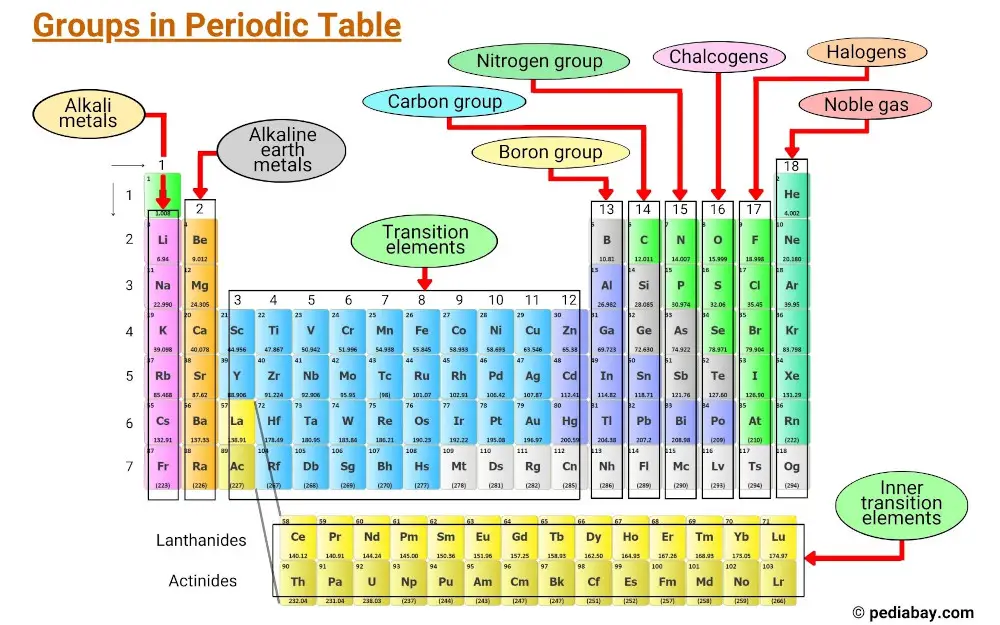
Elements are grouped together based on shared characteristics, including electron configurations and valence electron behavior.
The vertical columns in the periodic table are called groups and there are 18 groups in the periodic table, each with its own set of properties that determine how an element will interact with other elements and form compounds.
In this article, we will explore the various groups of the periodic table.
Let’s dive right into it!.
Table of contents:
- Group 1: Alkali metals
- Group 2: Alkaline earth metals
- Group 3 – 11: Transition metals
- Group 13: Boron group
- Group 14: Carbon group
- Group 15: Pnictogens
- Group 16: Chalcogens
- Group 17: Halogens
- Group 18: Noble gases
Group 1: Alkali metals
Group 1 of the periodic table is known as the alkali metals.
This group includes lithium, sodium, potassium, rubidium, cesium, and francium.
Alkali metals are known for their softness, low melting points, and high reactivity with water and air.
Alkali metals are highly reactive because they have only one electron in their outermost shell, which they readily give up to form positive ions. [1]
This makes them excellent conductors of electricity and useful in many applications, including batteries and industrial processes.
However, because of their high reactivity, alkali metals must be handled with care. They can ignite spontaneously in air and react explosively with water, releasing hydrogen gas. [2]
Group 2: Alkaline earth metals
Group 2 of the periodic table is known as the “alkaline earth metals.” This group includes six elements: beryllium, magnesium, calcium, strontium, barium, and radium.
These metals are called “alkaline” because they react with water to form alkaline (basic) solutions.
Alkaline earth metals have similar properties, including high melting and boiling points, low densities, and the ability to easily form ions with a +2 charge.
They are also highly reactive, especially with water and oxygen. However, unlike the alkali metals in Group 1, they are less reactive and have a higher melting point.
These elements are found in many everyday items, including bones (calcium), fireworks (strontium), and airplane parts (magnesium).
Alkaline earth metals are also used in industrial processes, such as the production of aluminum and steel.
Group 3 – 11: Transition metals
Group 3-11 of the periodic table is known as the transition metal group. These metals have unique characteristics that set them apart from other elements on the table.
One of the defining features of transition metals is their ability to form colored compounds.
This is because of their unique electron configurations, which allow them to absorb and reflect certain wavelengths of light. [3]
Transition metals are also known for their high melting and boiling points, as well as their strength and durability.
They are often used in building materials, electrical wiring, and even jewelry.
Group 13: Boron group
Group 13 of the periodic table is known as the Boron group, and it includes the elements Boron (B), Aluminum (Al), Gallium (Ga), Indium (In), Thallium (Tl) and Nihonium (Nh).
These elements share certain characteristics that make them chemically similar to one another.
Boron is the only metalloid in this group, which means that it has properties of both metals and nonmetals.
Group 14: Carbon group
Group 14 of the periodic table is known as the Carbon group and it includes carbon (C), silicon (Si), germanium (Ge), tin (Sn), lead (Pb) and Flerovium (Fl).
These elements have similar properties because they all have four valence electrons.
Carbon is a very important element because it is the basis of all organic molecules, including the building blocks of life such as proteins and DNA. [4]
Group 15: Pnictogens
Group 15 of the periodic table is also known as the Nitrogen Group (or pnictogens).
This group contains 6 elements: nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), bismuth (Bi) and moscovium (Mc).
The elements in Group 15 have five valence electrons in their outermost electron shell, which means they tend to form three covalent bonds to complete their octet.
They also have a tendency to gain three electrons to form anions. The elements become less non-metallic and more metallic down the group.
Group 16: Chalcogens
Group 16 of the periodic table is known as the oxygen group. It includes the elements oxygen (O), sulfur (S), selenium (Se), tellurium (Te), polonium (Po) and livermorium (Lv).
These elements share certain characteristics, including having six valence electrons in their outermost energy level.
Oxygen is the most abundant element in this group and is essential for life, as it is a key component in water and many organic molecules.
Elements in the oxygen group can form compounds with many other elements, including metals and nonmetals.
Group 17: Halogens
Group 17 of the periodic table is also known as the halogen group. It is made up of six elements: fluorine, chlorine, bromine, iodine, astatine and tennessine.
All halogens have seven electrons in their outermost shell, also known as the valence shell. This makes them highly reactive, as they only need one more electron to complete their outer shell and become stable.
Another important feature of halogens is that they are all nonmetals, which means that they do not conduct electricity and are not malleable or ductile like metals.
They also exist in all three states of matter at room temperature, with fluorine and chlorine being gases, bromine a liquid, and iodine and astatine solids.
Group 18: Noble gases
Group 18 of the periodic table is also known as the noble gases.
The noble gases include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn).
These gases are called noble because they are very stable and do not react easily with other elements.
The noble gases are located in the far right column of the periodic table, and they all have a full outer shell of electrons, making them very unreactive.
They are also odorless, colorless, and have low boiling and melting points.
External resources:
- Elements Organized by Group. (2015, July 26). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Descriptive_Chemistry/Elements_Organized_by_Group
- O. (n.d.). 2.5 The Periodic Table – Chemistry. 2.5 the Periodic Table – Chemistry. https://iu.pressbooks.pub/openstaxchemistry/chapter/2-5-the-periodic-table/
- Group (periodic table) – Wikipedia. (2018, September 15). Group (Periodic Table) – Wikipedia. https://en.wikipedia.org/wiki/Group_(periodic_table)
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.