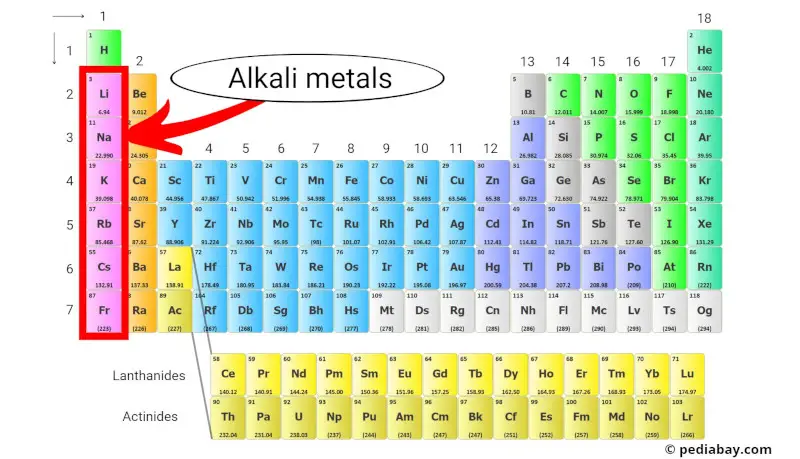
Alkali metals are a group of chemical elements located in the first column of the periodic table. This group includes lithium, sodium, potassium, rubidium, cesium, and francium.
Alkali metals are soft and have low melting and boiling points, and they are commonly used in batteries, alloys, and various chemical reactions.
Let’s explore more about the Alkali metals of the periodic table.
Table of contents:
- What are alkali metals?
- Why are alkali metals called so?
- Reactivity order of alkali metals
- List of alkali metals and their electron configurations
- Periodic trends of alkali metals
- Summary
What are alkali metals?

The alkali metals are a group of chemical elements in the periodic table that belong to Group 1.
These elements are known for their softness, low melting points, high reactivity, and ability to form alkaline solutions when they react with water.
Some of the key properties of alkali metals include:
- They have low electronegativities and ionization energies, which means that they tend to lose electrons easily and form cations.
- They have low melting and boiling points, which makes them relatively soft and easy to melt.
- They have high reactivity with water and oxygen, which makes them reactive in air and water.
- They are good conductors of heat and electricity.
Why are alkali metals called so?

Alkali metals are called so because they are able to form strong alkaline (basic) solutions when they react with water. [1]
When these metals come into contact with water, they release hydrogen gas and form hydroxide ions, which give the solution a basic pH.
Explanation:
The reactivity of alkali metals with water is due to their low ionization energies, [2] which means they are able to easily lose electrons and form positive ions (cations).
When they come into contact with water, they undergo a chemical reaction in which they lose electrons and react with water molecules to form hydroxide ions and hydrogen gas.
This reaction releases a lot of heat, which can cause the hydrogen gas to ignite and explode.
Reactivity order of alkali metals
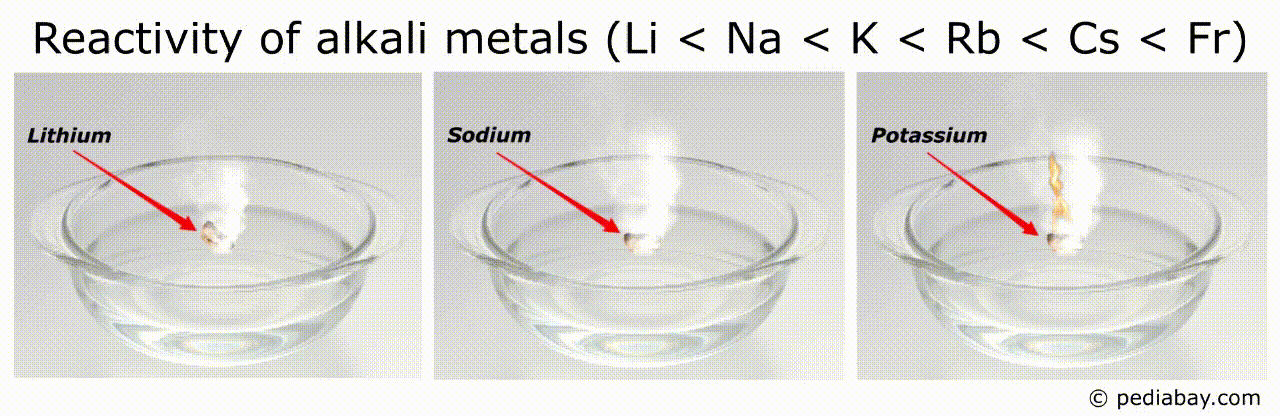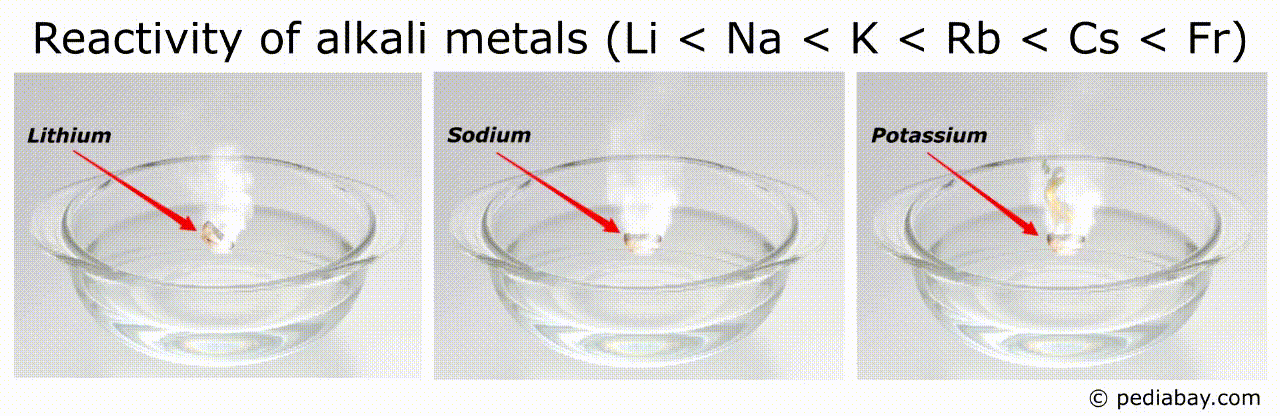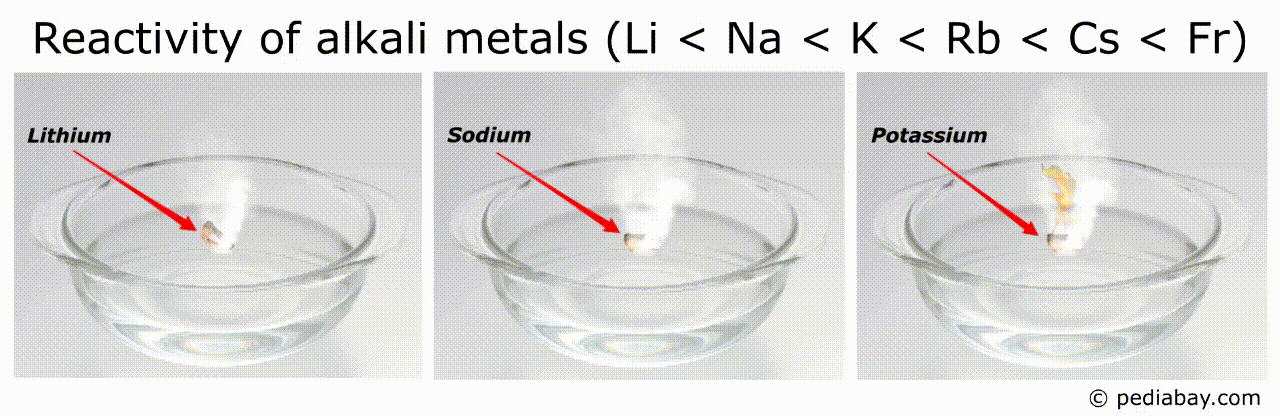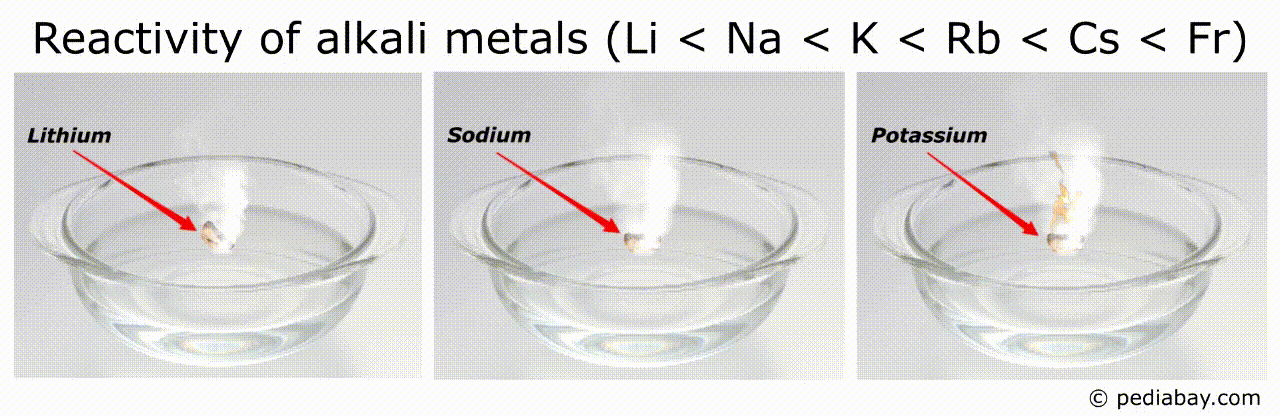
The general trend for reactivity order of alkali metals is that it increases as you move down the group from lithium (Li) to francium (Fr).
This means that francium is the most reactive of all the alkali metals, while lithium is the least reactive.
The reason for this trend is that, as you move down the group, the atomic radius of the elements increases, while the ionization energy decreases.
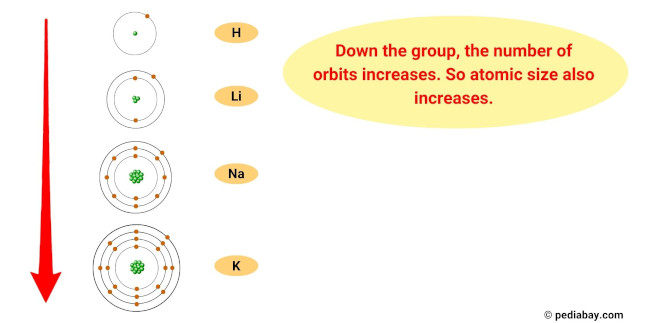
This means that the outermost electron of the atoms is further from the nucleus and held less tightly, making it easier for the element to lose this electron and become a cation.
Francium, being the largest and least tightly bound of the group, is therefore the most reactive.
This reactivity order has important implications for the properties and behavior of alkali metals.
For example, the most reactive metals (such as francium and cesium) can ignite spontaneously in air or water, making them very dangerous to handle. [3]
Conversely, the least reactive metals (such as lithium) are more stable and can be used in applications that require a more stable metal.
List of alkali metals and their electron configurations
The alkali metals and their electron configurations are given below.
| Element | Electron configuration |
| Lithium (Li) | [He] 2s1 |
| Sodium (Na) | [Ne] 3s1 |
| Potassium (K) | [Ar] 4s1 |
| Rubidium (Rb) | [Kr] 5s1 |
| Cesium (Cs) | [Xe] 6s1 |
| Francium (Fr) | [Rn] 7s1 |
Periodic trends of alkali metals
The periodic trends of alkali metals are mentioned below:
- Valency trend: The valency of alkali metals is always +1, meaning they tend to lose one electron to form a stable cation. So there is no change in valency of alkali metals as we move down the group. They all have the same valency (i.e 1).
- Atomic size trend: The atomic size of alkali metals increases as you move down the group, meaning that the atoms get larger. This is because as you add more energy levels, the size of the electron cloud increases.
- Metallic character trend: The metallic character of alkali metals increases as you move down the group. This is because the metals become more electropositive and are better able to donate electrons to form metallic bonds.
- Electronegativity trend: The electronegativity of alkali metals decreases as you move down the group. This is because the atoms get larger and the outer electrons are further away from the nucleus, so they are less attracted to it.
- Electron affinity trend: The electron affinity of alkali metals decreases as you move down the group. This is because down the group, the size of atoms increases which decreases the tendency of an atom to accept the electron. (Read more about the electron affinity trend from here, for detailed understanding).
- Ionization energy trend: The ionization energy of alkali metals decreases as you move down the group. This means that it is easier to remove an electron from the outermost shell of the atom, as the electrons are further from the nucleus and are held less tightly.
Summary
Alkali metals are a group of chemical elements known for their softness, low melting points, high reactivity, and ability to form alkaline solutions when they react with water.
The reactivity order of alkali metals increases as you move down the group from lithium to francium due to the increase in atomic radius and decrease in ionization energy.
The periodic trends of alkali metals include an increase in atomic size and metallic character as you move down the group, and a decrease in electronegativity, electron affinity, and ionization energy.
The valency of alkali metals is always +1, and their electron configurations follow a pattern of [noble gas] ns1.
External resources:
- Information on Alkali Metals – Stanford Environmental Health & Safety. (n.d.). Information on Alkali Metals – Stanford Environmental Health & Safety. http://ehs.stanford.edu/reference/information-alkali-metals
- Alkali Metal Reactivity | Chemdemos. (n.d.). Alkali Metal Reactivity | Chemdemos. http://chemdemos.uoregon.edu/demos/Alkali-Metal-Reactivity
- Alkali metal – Wikipedia. (2012, March 24). Alkali Metal – Wikipedia. https://en.wikipedia.org/wiki/Alkali_metal
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.