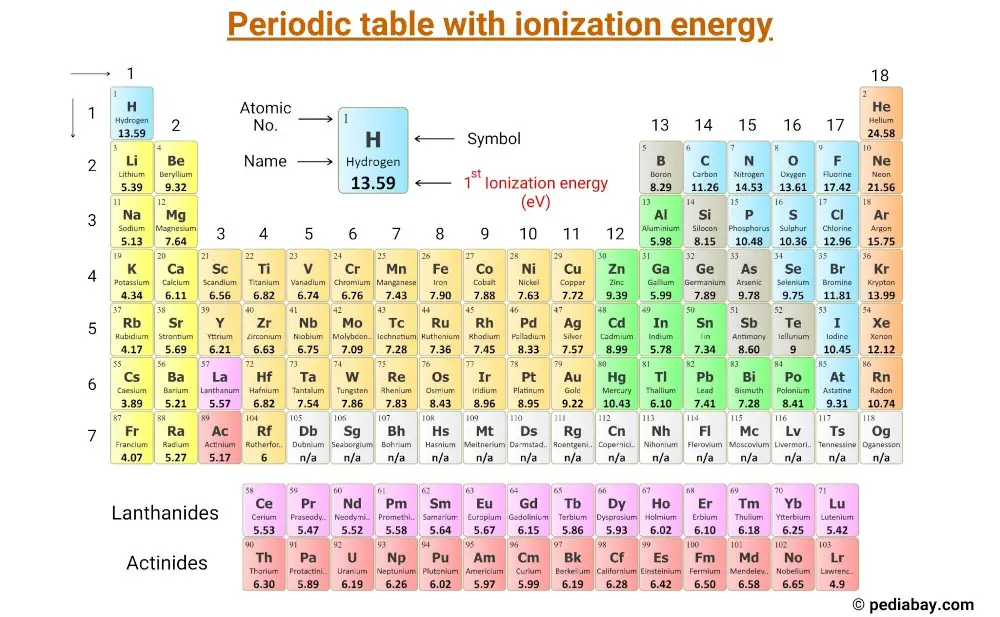
This is a periodic table with ionization energy of elements labeled on it.
The ionization energy is measured in joules (J) or electron volts (eV). The ionization energy values mentioned in the above periodic table are the First Ionization energy and are given in electron volts (eV).
In case you don’t know what ionization energy is, then here is a brief explanation on it.
What is Ionization energy?
Ionization energy is the minimum energy required to remove an electron from the gaseous atom or ion.
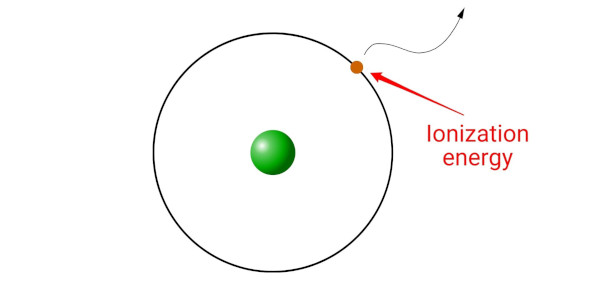
(Simple explanation: Ionization energy is like a key that unlocks the electron from its orbit. Without this key, the electron cannot escape. The ionization energy is the minimum amount of energy needed to unlock the electron and make it leave the atom or ion.)
List of elements with their Ionization energy
Here is a table of elements with their first ionization energy values (in eV, electron volt).
| Elements | First Ionization Energy (eV) |
| 1 | Hydrogen (H) | 13.59 eV |
| 2 | Helium (He) | 24.58 eV |
| 3 | Lithium (Li) | 5.39 eV |
| 4 | Beryllium (Be) | 9.32 eV |
| 5 | Boron (B) | 8.29 eV |
| 6 | Carbon (C) | 11.26 eV |
| 7 | Nitrogen (N) | 14.53 eV |
| 8 | Oxygen (O) | 13.61 eV |
| 9 | Fluorine (F) | 17.42 eV |
| 10 | Neon (Ne) | 21.56 eV |
| 11 | Sodium (Na) | 5.13 eV |
| 12 | Magnesium (Mg) | 7.64 eV |
| 13 | Aluminum (Al) | 5.98 eV |
| 14 | Silicon (Si) | 8.15 eV |
| 15 | Phosphorus (P) | 10.48 eV |
| 16 | Sulfur (S) | 10.36 eV |
| 17 | Chlorine (Cl) | 12.96 eV |
| 18 | Argon (Ar) | 15.75 eV |
| 19 | Potassium (K) | 4.34 eV |
| 20 | Calcium (Ca) | 6.11 eV |
| 21 | Scandium (Sc) | 6.56 eV |
| 22 | Titanium (Ti) | 6.82 eV |
| 23 | Vanadium (V) | 6.74 eV |
| 24 | Chromium (Cr) | 6.76 eV |
| 25 | Manganese (Mn) | 7.43 eV |
| 26 | Iron (Fe) | 7.90 eV |
| 27 | Cobalt (Co) | 7.88 eV |
| 28 | Nickel (Ni) | 7.63 eV |
| 29 | Copper (Cu) | 7.72 eV |
| 30 | Zinc (Zn) | 9.39 eV |
| 31 | Gallium (Ga) | 5.99 eV |
| 32 | Germanium (Ge) | 7.89 eV |
| 33 | Arsenic (As) | 9.78 eV |
| 34 | Selenium (Se) | 9.75 eV |
| 35 | Bromine (Br) | 11.81 eV |
| 36 | Krypton (Kr) | 13.99 eV |
| 37 | Rubidium (Rb) | 4.17 eV |
| 38 | Strontium (Sr) | 5.69 eV |
| 39 | Yttrium (Y) | 6.21 eV |
| 40 | Zirconium (Zr) | 6.63 eV |
| 41 | Niobium (Nb) | 6.75 eV |
| 42 | Molybdenum (Mo) | 7.09 eV |
| 43 | Technetium (Tc) | 7.28 eV |
| 44 | Ruthenium (Ru) | 7.36 eV |
| 45 | Rhodium (Rh) | 7.45 eV |
| 46 | Palladium (Pd) | 8.33 eV |
| 47 | Silver (Ag) | 7.57 eV |
| 48 | Cadmium (Cd) | 8.99 eV |
| 49 | Indium (In) | 5.78 eV |
| 50 | Tin (Sn) | 7.34 eV |
| 51 | Antimony (Sb) | 8.60 eV |
| 52 | Tellurium (Te) | 9 eV |
| 53 | Iodine (I) | 10.45 eV |
| 54 | Xenon (Xe) | 12.12 eV |
| 55 | Caesium (Cs) | 3.89 eV |
| 56 | Barium (Ba) | 5.21 eV |
| 57 | Lanthanum | 5.57 eV |
| 58 | Cerium (Ce) | 5.53 eV |
| 59 | Praseodymium (Pr) | 5.47 eV |
| 60 | Neodymium (Nd) | 5.52 eV |
| 61 | Promethium (Pm) | 5.58 eV |
| 62 | Samarium (Sm) | 5.64 eV |
| 63 | Europium (Eu) | 5.67 eV |
| 64 | Gadolinium (Gd) | 6.15 eV |
| 65 | Terbium (Tb) | 5.86 eV |
| 66 | Dysprosium (Dy) | 5.93 eV |
| 67 | Holmium (Ho) | 6.02 eV |
| 68 | Erbium (Er) | 6.10 eV |
| 69 | Thulium (Tm) | 6.18 eV |
| 70 | Ytterbium (Yb) | 6.25 eV |
| 71 | Lutenium (Lu) | 5.42 eV |
| 72 | Hafnium (Hf) | 6.82 eV |
| 73 | Tantalum (Ta) | 7.54 eV |
| 74 | Tungsten (W) | 7.86 eV |
| 75 | Rhenium (Re) | 7.83 eV |
| 76 | Osmium (Os) | 8.43 eV |
| 77 | Iridium (Ir) | 8.96 eV |
| 78 | Platinum (Pt) | 8.95 eV |
| 79 | Gold (Au) | 9.22 eV |
| 80 | Mercury (Hg) | 10.43 v |
| 81 | Thallium (Tl) | 6.10 eV |
| 82 | Lead (Pb) | 7.41 eV |
| 83 | Bismuth (Bi) | 7.28 eV |
| 84 | Polonium (Po) | 8.41 eV |
| 85 | Astatine (At) | 9.31 eV |
| 86 | Radon (Rn) | 10.74 eV |
| 87 | Francium (Fr) | 4.07 eV |
| 88 | Radium (Ra) | 5.27 eV |
| 89 | Actinium (Ac) | 5.17 eV |
| 90 | Thorium (Th) | 6.30 eV |
| 91 | Protactinium (Pa) | 5.89 eV |
| 92 | Uranium (U) | 6.19 eV |
| 93 | Neptunium (Np) | 6.26 eV |
| 94 | Plutonium (Pu) | 6.02 eV |
| 95 | Americium (Am) | 5.97 eV |
| 96 | Curium (Cm) | 5.99 eV |
| 97 | Berkelium (Bk) | 6.19 eV |
| 98 | Californium (Cf) | 6.28 eV |
| 99 | Einsteinium (Es) | 6.42 eV |
| 100 | Fermium (Fm) | 6.50 v |
| 101 | Mendelevium (Md) | 6.58 eV |
| 102 | Nobelium (No) | 6.65 eV |
| 103 | Lawrencium (Lr) | 4.9 eV |
| 104 | Rutherfordium (Rf) | 6 eV |
| 105 | Dubnium (Db) | – |
| 106 | Seaborgium (Sg) | – |
| 107 | Bohrium (Bh) | – |
| 108 | Hassium (Hs) | – |
| 109 | Meitnerium (Mt) | – |
| 110 | Darmstadtium (Ds) | – |
| 111 | Roentgenium (Rg) | – |
| 112 | Copernicium (Cn) | – |
| 113 | Nihonium (Nh) | – |
| 114 | Flerovium (Fl) | – |
| 115 | Moscovium (Mc) | – |
| 116 | Livermorium (Lv) | – |
| 117 | Tennessine (Ts) | – |
| 118 | Oganesson (Og) | – |
External resources:
- First ionisation energy. (n.d.). Chemguide. https://www.chemguide.co.uk/atoms/properties/ies.html
- NIST Atomic Spectra Database Ionization Energies Form. (n.d.). National Institute of Standards and Technology. https://physics.nist.gov/PhysRefData/ASD/ionEnergy.html
- Ionization energies of the elements (data page) – Wikipedia. (2020, September 1). Ionization Energies of the Elements (Data Page) – Wikipedia. https://en.wikipedia.org/wiki/Ionization_energies_of_the_elements_(data_page)
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.

