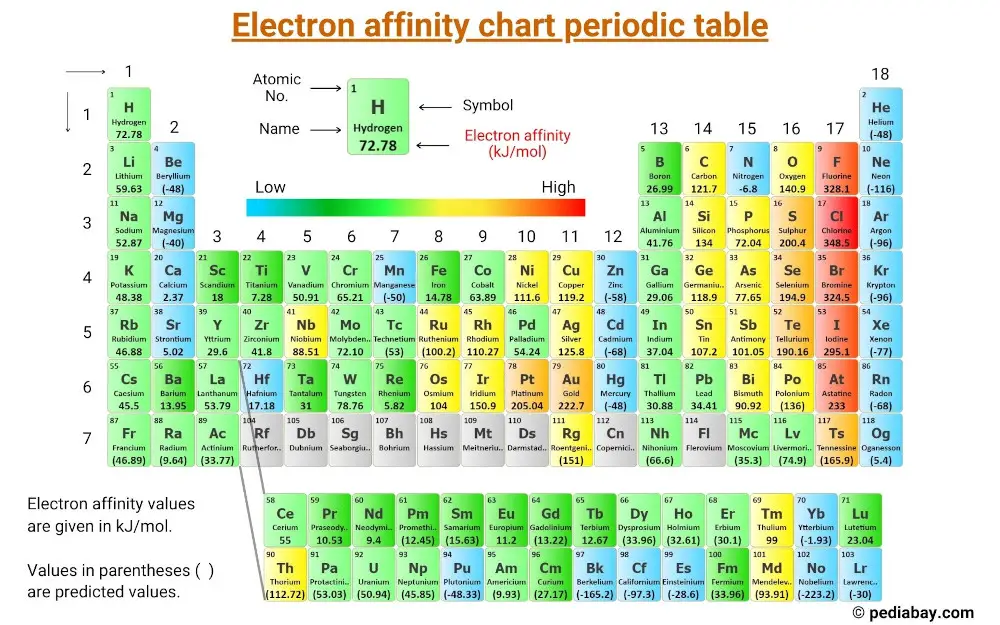
This is a periodic table with electron affinity of elements labeled on it.
[Note: The values of electron affinity are given in kJ/mol. Values in parentheses ( ) are predicted values].
In case you don’t know what electron affinity is, then here is a brief explanation on it.
What is electron affinity?
Electron affinity is a measure of the energy change that occurs when an electron is added to the outermost shell of an isolated gaseous atom.
The amount of energy change, also known as ΔE, can be positive, negative, or zero.
A positive value of ΔE indicates that energy is absorbed during the process, while a negative value of ΔE indicates that energy is released.
The sign of Electron Affinity (EEA) is opposite to the sign of energy change (ΔE), with a positive EEA value corresponding to a negative ΔE value and vice versa.
The higher the positive value of EEA, the more energy is released when an electron is added to the neutral atom.
For example: Halogens have high positive values of EEA, indicating that they release a lot of energy when an electron is added to their outermost shell.
Electron affinity of All the Elements of Periodic Table
| Elements | Electron affinity (kJ/mol) |
| 1 | Hydrogen (H) | 72.78 |
| 2 | Helium (He) | (-48) |
| 3 | Lithium (Li) | 59.63 |
| 4 | Beryllium (Be) | (-48) |
| 5 | Boron (B) | 26.99 |
| 6 | Carbon (C) | 121.77 |
| 7 | Nitrogen (N) | -6.8 |
| 8 | Oxygen (O) | 140.98 |
| 9 | Fluorine (F) | 328.16 |
| 10 | Neon (Ne) | (-116) |
| 11 | Sodium (Na) | 52.87 |
| 12 | Magnesium (Mg) | (-40) |
| 13 | Aluminum (Al) | 41.76 |
| 14 | Silicon (Si) | 134.06 |
| 15 | Phosphorus (P) | 72.04 |
| 16 | Sulfur (S) | 200.4 |
| 17 | Chlorine (Cl) | 348.57 |
| 18 | Argon (Ar) | (-96) |
| 19 | Potassium (K) | 48.38 |
| 20 | Calcium (Ca) | 2.37 |
| 21 | Scandium (Sc) | 18 |
| 22 | Titanium (Ti) | 7.28 |
| 23 | Vanadium (V) | 50.91 |
| 24 | Chromium (Cr) | 65.21 |
| 25 | Manganese (Mn) | (-50) |
| 26 | Iron (Fe) | 14.78 |
| 27 | Cobalt (Co) | 63.89 |
| 28 | Nickel (Ni) | 111.65 |
| 29 | Copper (Cu) | 119.23 |
| 30 | Zinc (Zn) | (-58) |
| 31 | Gallium (Ga) | 29.06 |
| 32 | Germanium (Ge) | 118.93 |
| 33 | Arsenic (As) | 77.65 |
| 34 | Selenium (Se) | 194.95 |
| 35 | Bromine (Br) | 324.53 |
| 36 | Krypton (Kr) | (-96) |
| 37 | Rubidium (Rb) | 46.88 |
| 38 | Strontium (Sr) | 5.02 |
| 39 | Yttrium (Y) | 29.6 |
| 40 | Zirconium (Zr) | 41.8 |
| 41 | Niobium (Nb) | 88.51 |
| 42 | Molybdenum (Mo) | 72.10 |
| 43 | Technetium (Tc) | (53) |
| 44 | Ruthenium (Ru) | (100.27) |
| 45 | Rhodium (Rh) | 110.27 |
| 46 | Palladium (Pd) | 54.24 |
| 47 | Silver (Ag) | 125.86 |
| 48 | Cadmium (Cd) | (-68) |
| 49 | Indium (In) | 37.04 |
| 50 | Tin (Sn) | 107.29 |
| 51 | Antimony (Sb) | 101.05 |
| 52 | Tellurium (Te) | 190.16 |
| 53 | Iodine (I) | 295.15 |
| 54 | Xenon (Xe) | (-77) |
| 55 | Caesium (Cs) | 45.5 |
| 56 | Barium (Ba) | 13.95 |
| 57 | Lanthanum | 53.79 |
| 58 | Cerium (Ce) | 55 |
| 59 | Praseodymium (Pr) | 10.53 |
| 60 | Neodymium (Nd) | 9.4 |
| 61 | Promethium (Pm) | (12.45) |
| 62 | Samarium (Sm) | (15.63) |
| 63 | Europium (Eu) | 11.2 |
| 64 | Gadolinium (Gd) | (13.22) |
| 65 | Terbium (Tb) | 12.67 |
| 66 | Dysprosium (Dy) | (33.96) |
| 67 | Holmium (Ho) | (32.61) |
| 68 | Erbium (Er) | (30.1) |
| 69 | Thulium (Tm) | 99 |
| 70 | Ytterbium (Yb) | (-1.93) |
| 71 | Lutenium (Lu) | 23.04 |
| 72 | Hafnium (Hf) | 17.18 |
| 73 | Tantalum (Ta) | 31 |
| 74 | Tungsten (W) | 78.76 |
| 75 | Rhenium (Re) | 5.82 |
| 76 | Osmium (Os) | 104 |
| 77 | Iridium (Ir) | 150.94 |
| 78 | Platinum (Pt) | 205.04 |
| 79 | Gold (Au) | 222.75 |
| 80 | Mercury (Hg) | (-48) |
| 81 | Thallium (Tl) | 30.88 |
| 82 | Lead (Pb) | 34.41 |
| 83 | Bismuth (Bi) | 90.92 |
| 84 | Polonium (Po) | (136) |
| 85 | Astatine (At) | 233 |
| 86 | Radon (Rn) | (-68) |
| 87 | Francium (Fr) | (46.89) |
| 88 | Radium (Ra) | (9.64) |
| 89 | Actinium (Ac) | (33.77) |
| 90 | Thorium (Th) | (112.72) |
| 91 | Protactinium (Pa) | (53.03) |
| 92 | Uranium (U) | (50.94) |
| 93 | Neptunium (Np) | (45.85) |
| 94 | Plutonium (Pu) | (-48.33) |
| 95 | Americium (Am) | (9.93) |
| 96 | Curium (Cm) | (27.17) |
| 97 | Berkelium (Bk) | (-165.24) |
| 98 | Californium (Cf) | (-97.31) |
| 99 | Einsteinium (Es) | (-28.6) |
| 100 | Fermium (Fm) | (33.96) |
| 101 | Mendelevium (Md) | (93.91) |
| 102 | Nobelium (No) | (-223.22) |
| 103 | Lawrencium (Lr) | (-30.04) |
| 104 | Rutherfordium (Rf) | unknown |
| 105 | Dubnium (Db) | unknown |
| 106 | Seaborgium (Sg) | unknown |
| 107 | Bohrium (Bh) | unknown |
| 108 | Hassium (Hs) | unknown |
| 109 | Meitnerium (Mt) | unknown |
| 110 | Darmstadtium (Ds) | unknown |
| 111 | Roentgenium (Rg) | (151) |
| 112 | Copernicium (Cn) | unknown |
| 113 | Nihonium (Nh) | (66.6) |
| 114 | Flerovium (Fl) | unknown |
| 115 | Moscovium (Mc) | (35.3) |
| 116 | Livermorium (Lv) | (74.9) |
| 117 | Tennessine (Ts) | (165.9) |
| 118 | Oganesson (Og) | (5.403) |
External resources:
- Electron affinity | physics. (n.d.). Encyclopedia Britannica. https://www.britannica.com/science/electron-affinity
- Union of Pure and Applied Chemistry (IUPAC), T. I. (n.d.). IUPAC – electron affinity (E01977). IUPAC – Electron Affinity (E01977). https://goldbook.iupac.org/terms/view/E01977
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.
