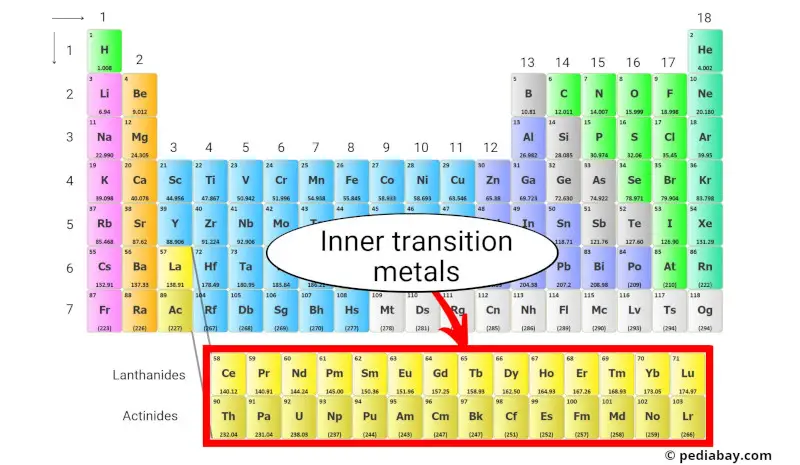
Inner transition metals are a group of chemical elements that are located in the bottom two rows of the periodic table, just below the main block of transition metals.
The inner transition metals are divided into two groups:
- Lanthanides, which have atomic numbers ranging from 57 to 71, and
- Actinides, which have atomic numbers ranging from 89 to 103.
Let’s explore more about the Inner transition elements of the periodic table.
Table of contents:
- What are inner transition metals? And why are they called so?
- List of inner transition metals
- Facts about Inner transition metals
- Properties of Inner transition metals
- Uses of Inner transition metals
- Summary
What are inner transition metals? And why are they placed at the bottom?
The inner transition metals are the group of elements that are placed at the bottom of the periodic table.
These inner transition metals are the part of transition metals only, and they also have some similar properties as that of transition metals. But they are placed in the inner section as an extension of group 3.
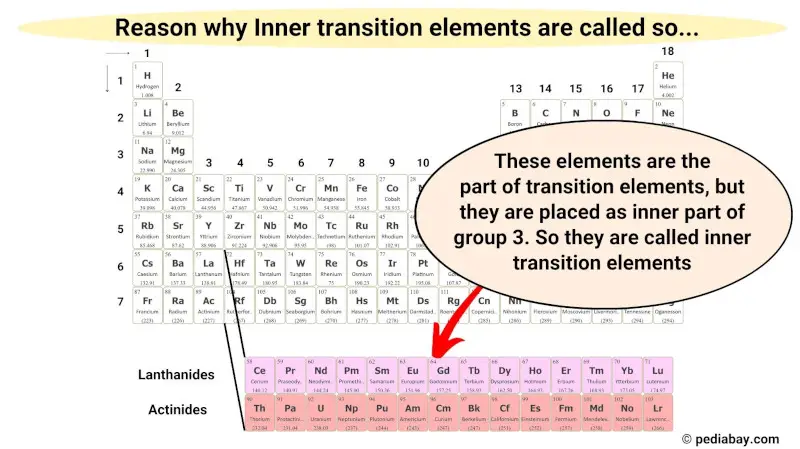
Hence these metallic elements are known as inner transition metals.
Why are they placed at the bottom of the periodic table?
If the inner transition metals are placed as an extension of group 3, then the periodic table would look very long (as shown below).
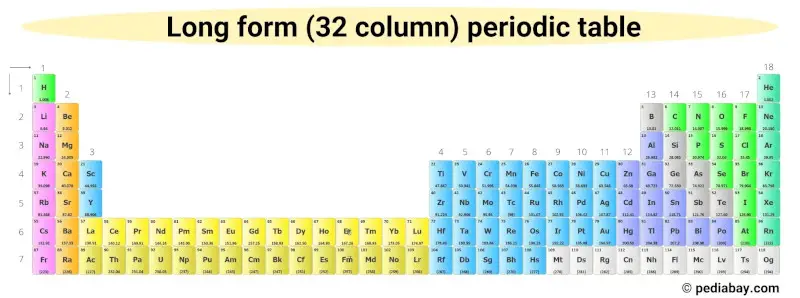
So in order to make it fit on an A4 size paper, the 2 rows of inner transition metals are placed at the bottom.
Also, these inner transition elements have their valence electrons in the f-orbital, due to which these elements show similar chemical properties.
Hence because of these reasons, the inner transition metals are placed at the bottom of the periodic table.
List of inner transition metals
The list of inner transition metals is given below.
| Atomic number | Symbol | Name and symbol of element |
| 57 | La | Lanthanum |
| 58 | Ce | Cerium |
| 59 | Pr | Praseodymium |
| 60 | Nd | Neodymium |
| 61 | Pm | Promethium |
| 62 | Sm | Samarium |
| 63 | Eu | Europium |
| 64 | Gd | Gadolinium |
| 65 | Tb | Terbium |
| 66 | Dy | Dysprosium |
| 67 | Ho | Holmium |
| 68 | Er | Erbium |
| 69 | Tm | Thulium |
| 70 | Yb | Ytterbium |
| 71 | Lu | Lutetium |
| 89 | Ac | Actinium |
| 90 | Th | Thorium |
| 91 | Pa | Protactinium |
| 92 | U | Uranium |
| 93 | Np | Neptunium |
| 94 | Pu | Plutonium |
| 95 | Am | Americium |
| 96 | Cm | Curium |
| 97 | Bk | Berkelium |
| 98 | Cf | Californium |
| 99 | Es | Einsteinium |
| 100 | Fm | Fermium |
| 101 | Md | Mendelevium |
| 102 | No | Nobelium |
| 103 | Lr | Lawrencium |
Facts about Inner transition metals
Here are some interesting facts about inner transition metals:
- There are two types of inner transition metals: lanthanides and actinides. Lanthanides are the 15 elements with atomic numbers ranging from 57 (lanthanum) to 71 (lutetium), while actinides are the 15 elements with atomic numbers ranging from 89 (actinium) to 103 (lawrencium).
- Inner transition metals have unique electronic configurations due to the presence of f-orbitals. This results in unusual chemical and physical properties, such as high melting and boiling points, complex ion formation, and paramagnetism.
- Most inner transition metals are radioactive, and some have been used in nuclear reactors and weapons.
- Inner transition metals are important in technology and industry. For example, neodymium and samarium are used to make powerful magnets, [1] and cerium is used in catalytic converters to reduce emissions from vehicles. [2]
- The lanthanides are also known as the “rare earth elements” because they are relatively scarce in the earth’s crust. However, they are not actually rare, and are found in many minerals.
- The actinides are all radioactive, and some, like uranium and plutonium, are important for nuclear energy and weapons. However, they also pose environmental and health risks due to their radioactivity.
- Inner transition metals have been known since ancient times, and were used for jewelry and decoration. However, they were not widely studied or understood until the 20th century.
Properties of Inner transition metals
Here are some properties of inner transition metals:
- Electronic configuration: Inner transition metals have partially filled f-orbitals, which gives them unique electronic configurations and unusual chemical and physical properties.
- High melting and boiling points: Inner transition metals have high melting and boiling points due to the strong metallic bonding resulting from the presence of unpaired electrons in the f-orbitals.
- Paramagnetism: Inner transition metals are paramagnetic, meaning that they are attracted to a magnetic field due to the presence of unpaired electrons in the f-orbitals.
- Complex ion formation: Inner transition metals can form complex ions due to the availability of empty f-orbitals, which can accommodate electrons from other atoms or molecules.
- Radioactivity: Many inner transition metals are radioactive due to the presence of unstable isotopes in their nuclei.
- Oxidation states: Inner transition metals exhibit a range of oxidation states, including high oxidation states, due to the availability of multiple valence electrons in the f-orbitals.
- Similar chemical properties: The lanthanides and actinides have similar chemical properties, which makes them difficult to separate from each other.
- Metallic luster: Inner transition metals are typically shiny and have a metallic luster due to their high reflectivity of light.
Uses of Inner transition metals
Inner transition metals have a wide range of uses due to their unique properties. Some of the uses of inner transition metals are:
- Nuclear energy: Many inner transition metals, especially actinides like uranium, are used as fuel for nuclear reactors and weapons.
- Magnets: Lanthanides like neodymium, samarium, and gadolinium are used to make powerful magnets for various applications, including computer hard drives, wind turbines, and MRI machines.
- Lighting: Lanthanides like europium and terbium are used to make phosphors for fluorescent and LED lighting. [3]
- Catalysis: Lanthanides like cerium are used as catalysts in many industrial processes, including petroleum refining and pollution control.
- Glassmaking: Lanthanides like erbium and ytterbium are used to color glass, while cerium is used to polish glass.
- Medical imaging: Lanthanides like gadolinium and terbium are used in contrast agents for MRI scans. [4]
- Electronics: Lanthanides like dysprosium and terbium are used in electronic devices, such as microwave filters and LCD displays.
- Defense: Inner transition metals are used in defense technologies, including nuclear weapons and armor-piercing ammunition.
Summary
Inner transition metals are a group of chemical elements located at the bottom two rows of the periodic table, just below the main block of transition metals.
They are divided into two groups: Lanthanides and Actinides, which have atomic numbers ranging from 57 to 71 and 89 to 103, respectively.
The placement of inner transition metals is at the bottom of the periodic table because their valence electrons are in the f-orbital, which shows similar chemical properties.
Inner transition metals have high melting and boiling points, complex ion formation, and paramagnetism. They also exhibit a range of oxidation states, including high oxidation states, due to the availability of multiple valence electrons in the f-orbitals.
External resources:
- Boudreaux, K. A. (n.d.). The Parts of the Periodic Table. The Parts of the Periodic Table. http://www.angelo.edu/faculty/kboudrea/periodic/trans_inner_transition.htm
- General Chemistry/Chemistries of Various Elements/Inner Transition Metals – Wikibooks, open books for an open world. (n.d.). General Chemistry/Chemistries of Various Elements/Inner Transition Metals – Wikibooks, Open Books for an Open World. https://en.wikibooks.org/wiki/General_Chemistry/Chemistries_of_Various_Elements/Inner_Transition_Metals
- Transition metal | Definition, Properties, Elements, & Facts. (n.d.). Encyclopedia Britannica. https://www.britannica.com/science/transition-metal
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.