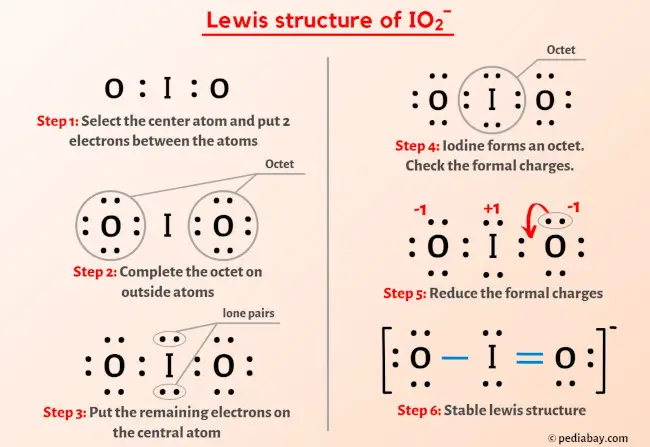
So you have seen the above image by now, right?
Let me explain the above image in short.
IO2– lewis structure has an Iodine atom (I) at the center which is surrounded by two Oxygen atoms (O). There is 1 single bond and 1 double bond between the Iodine atom (I) and each Oxygen atom (O). There are 2 lone pairs on double bonded Oxygen atom (O) and 3 lone pairs on single bonded Oxygen atom (O).
If you haven’t understood anything from the above image of IO2- lewis structure, then just stick with me and you will get the detailed step by step explanation on drawing a lewis structure of IO2- ion.
So let’s move to the steps of drawing the lewis structure of IO2- ion.
Steps of drawing IO2- lewis structure
Step 1: Find the total valence electrons in IO2- ion
In order to find the total valence electrons in IO2- ion, first of all you should know the valence electrons present in iodine atom as well as oxygen atom.
(Valence electrons are the electrons that are present in the outermost orbit of any atom.)
Here, I’ll tell you how you can easily find the valence electrons of iodine as well as oxygen using a periodic table.
Total valence electrons in IO2- ion
→ Valence electrons given by iodine atom:
Iodine is a group 17 element on the periodic table. [1] Hence the valence electrons present in iodine is 7.
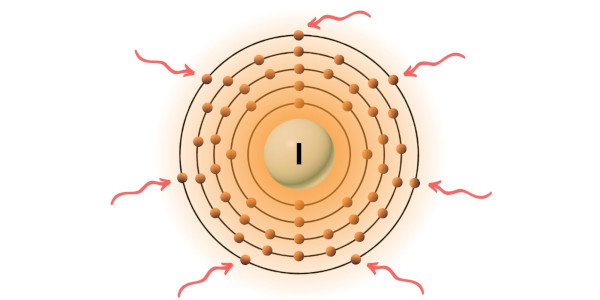
You can see the 7 valence electrons present in the iodine atom as shown in the above image.
→ Valence electrons given by oxygen atom:
Oxygen is group 16 element on the periodic table. [2] Hence the valence electrons present in oxygen is 6.
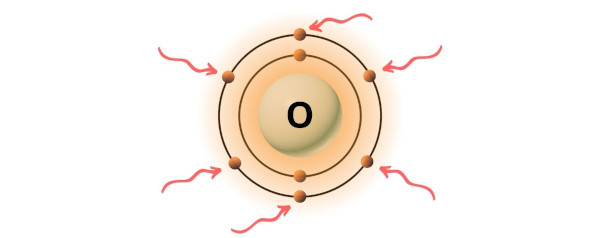
You can see the 6 valence electrons present in the oxygen atom as shown in the above image.
Hence,
Total valence electrons in IO2– ion = valence electrons given by 1 iodine atom + valence electrons given by 2 oxygen atoms + 1 more electron is added due to 1 negative charge = 7 + 6(2) + 1 = 20.
Step 2: Select the central atom
For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.
Now here the given ion is IO2- and it contains iodine atom (I) and oxygen atoms (O).
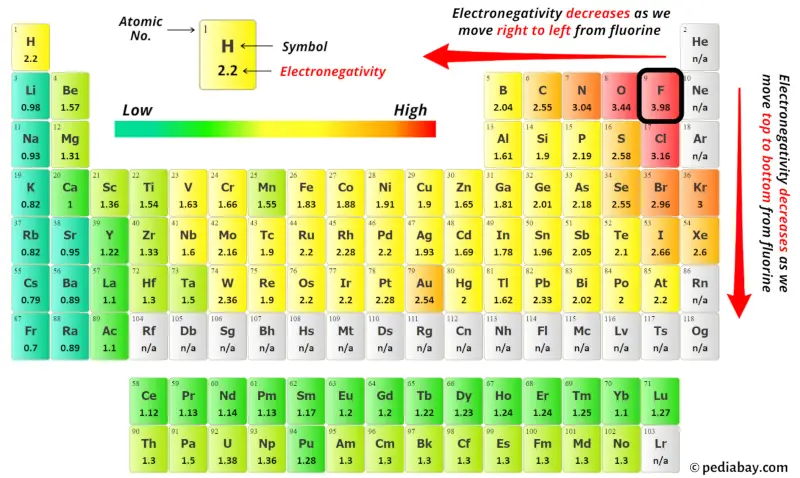
You can see the electronegativity values of iodine atom (I) and oxygen atom (O) in the above periodic table.
If we compare the electronegativity values of iodine (I) and oxygen (O) then the iodine atom is less electronegative.
So here the iodine atom (I) is the center atom and the oxygen atoms (O) are the outside atoms.
Step 3: Connect each atoms by putting an electron pair between them
Now in the IO2 molecule, you have to put the electron pairs between the iodine atom (I) and oxygen atoms (O).
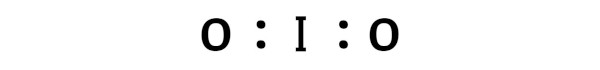
This indicates that the iodine (I) and oxygen (O) are chemically bonded with each other in a IO2 molecule.
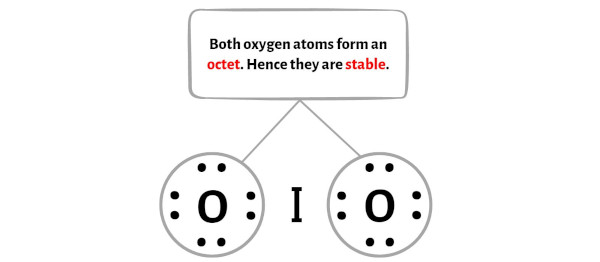
Step 4: Make the outer atoms stable. Place the remaining valence electrons pair on the central atom.
Now in this step, you have to check the stability of the outer atoms.
Here in the sketch of IO2 molecule, you can see that the outer atoms are oxygen atoms.
These outer oxygen atoms are forming an octet and hence they are stable.
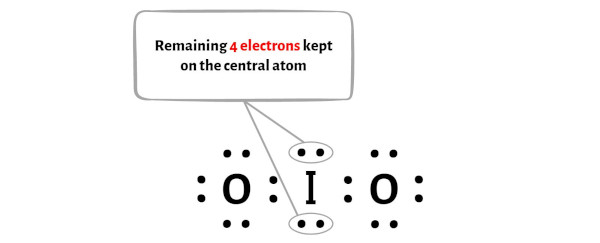
Also, in step 1 we have calculated the total number of valence electrons present in the IO2- ion.
The IO2- ion has a total 20 valence electrons and out of these, only 16 valence electrons are used in the above sketch.
So the number of electrons which are left = 20 – 16 = 4.
You have to put these 4 electrons on the central iodine atom in the above sketch of IO2 molecule.
Now let’s proceed to the next step.
Step 5: Check the octet on the central atom
In this step, you have to check whether the central iodine atom (I) is stable or not.
In order to check the stability of the central iodine (I) atom, we have to check whether it is forming an octet or not.
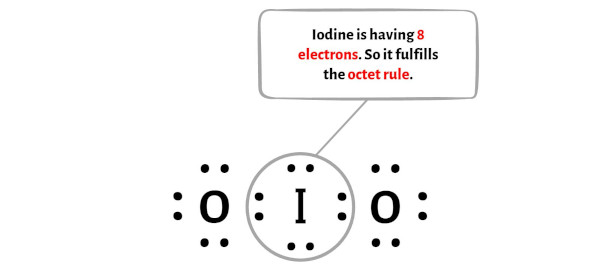
You can see from the above picture that the iodine atom is forming an octet. That means it has 8 electrons.
And hence the central iodine atom is stable.
Now let’s proceed to the final step to check whether the lewis structure of IO2- ion is stable or not.
Step 6: Check the stability of lewis structure
Now you have come to the final step in which you have to check the stability of lewis structure of IO2- ion.
The stability of lewis structure can be checked by using a concept of formal charge.
In short, now you have to find the formal charge on iodine (I) atom as well as oxygen (O) atoms present in the IO2- ion.
For calculating the formal charge, you have to use the following formula;
Formal charge = Valence electrons – (Bonding electrons)/2 – Nonbonding electrons
You can see the number of bonding electrons and nonbonding electrons for each atom of IO2 molecule in the image given below.
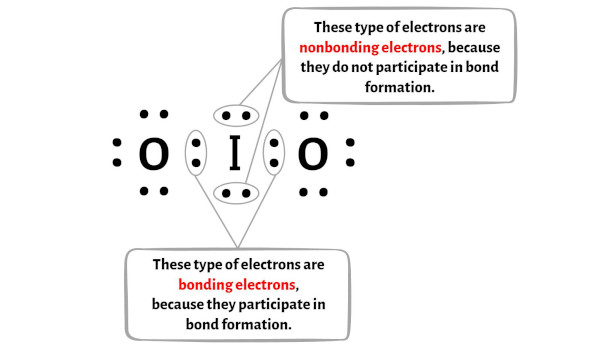
For Iodine (I) atom:
Valence electron = 7 (because iodine is in group 17)
Bonding electrons = 4
Nonbonding electrons = 4
For Oxygen (O) atom:
Valence electrons = 6 (because oxygen is in group 16)
Bonding electrons = 2
Nonbonding electrons = 6
| Formal charge | = | Valence electrons | – | (Bonding electrons)/2 | – | Nonbonding electrons | ||
| I | = | 7 | – | 4/2 | – | 4 | = | +1 |
| O | = | 6 | – | 2/2 | – | 6 | = | -1 |
From the above calculations of formal charge, you can see that the iodine (I) atom has +1 charge and both the oxygen (O) atoms have -1 charges.
Because of this reason, the above obtained lewis structure is not stable.
So we have to minimize these charges by shifting the electron pair from the oxygen atom to the iodine atom.
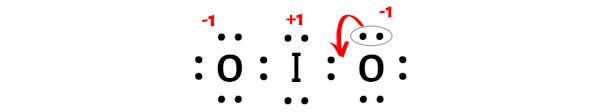
After shifting the electron pair from oxygen atom to iodine atom, the lewis structure of IO2 becomes more stable.
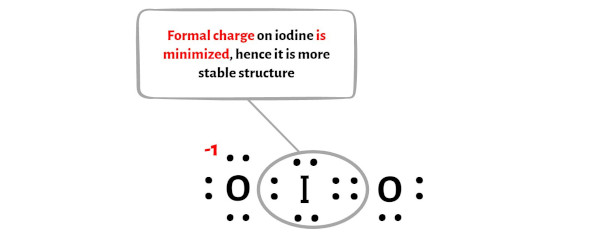
There is a -ve charge left on the oxygen atoms, which gives -1 formal charge on the IO2 molecule.
This overall -1 charge on the IO2 molecule is represented in the image given below.
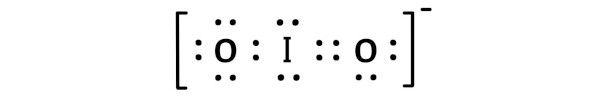
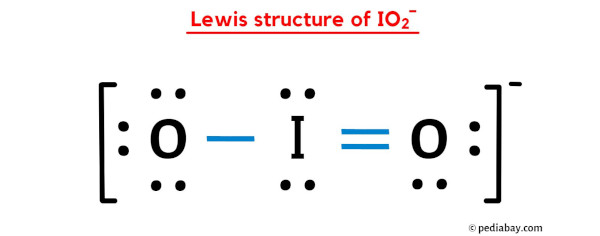
In the above lewis dot structure of IO2- ion, you can also represent each bonding electron pair (:) as a single bond (|). By doing so, you will get the following lewis structure of IO2- ion.
I hope you have completely understood all the above steps.
For more practice and better understanding, you can try other lewis structures listed below.
Try (or at least See) these lewis structures for better understanding:
| BI3 Lewis Structure | CH3I Lewis Structure |
| BrO- Lewis Structure | SeOF2 Lewis Structure |
| SBr6 Lewis Structure | IO3- Lewis Structure |
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.