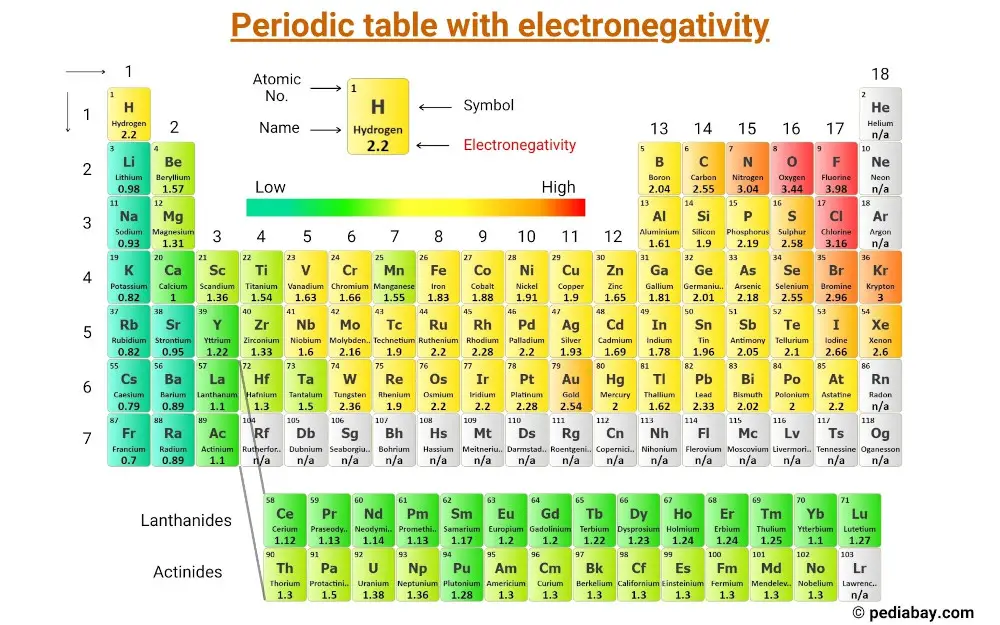
This is a periodic table with electronegativity of elements labeled on it.
(Note: Electronegativity has no unit. Linus Pauling was a scientist who designed a scale of electronegativity that ranks the elements with respect to each other. And this scale is known as Pauling electronegativity scale.) [1]
In case you don’t know what electronegativity is, then here is a brief explanation on it.
What is Electronegativity?
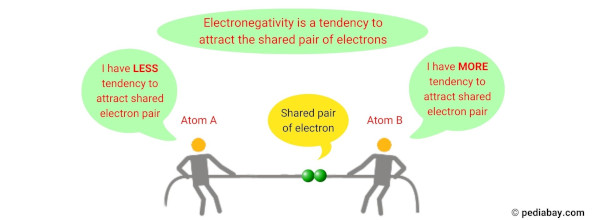
Electronegativity is a chemical property that describes the ability of an atom to attract electrons towards itself when it is part of a compound or molecule.
It is a measure of the relative attraction an atom has for shared electrons in a covalent bond.
Why is electronegativity important?
Electronegativity plays a crucial role in predicting the nature of chemical bonding, reactivity, and the physical properties of molecules.
It helps to explain why certain elements tend to form specific types of bonds, such as covalent or ionic bonds, and how those bonds affect the behavior of the resulting compound.
Electronegativity also helps to predict the polarity of a molecule and its interactions with other molecules, which is important in many fields, including chemistry, biology, and materials science.
What does higher electronegativity mean?
When an atom has a higher electronegativity, it means that it has a greater ability to attract electrons towards itself when it is part of a chemical compound or molecule.
This leads to the formation of polar covalent bonds, where the electrons are not shared equally between the atoms.
The atom with the higher electronegativity attracts the electrons closer to itself, resulting in a partial negative charge, while the other atom in the bond has a partial positive charge.
This polarity affects the physical and chemical properties of the compound, such as its boiling and melting points, solubility, and reactivity.
Examples of elements with high electronegativity include fluorine, oxygen, and nitrogen.
External resources:
- Boudreaux, K. A. (n.d.). The Parts of the Periodic Table. The Parts of the Periodic Table. https://www.angelo.edu/faculty/kboudrea/periodic/trends_electronegativity.htm
- Electronegativity – an overview | ScienceDirect Topics. (n.d.). Electronegativity – an Overview | ScienceDirect Topics. https://doi.org/10.1016/B978-0-12-803478-1.00012-1
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.