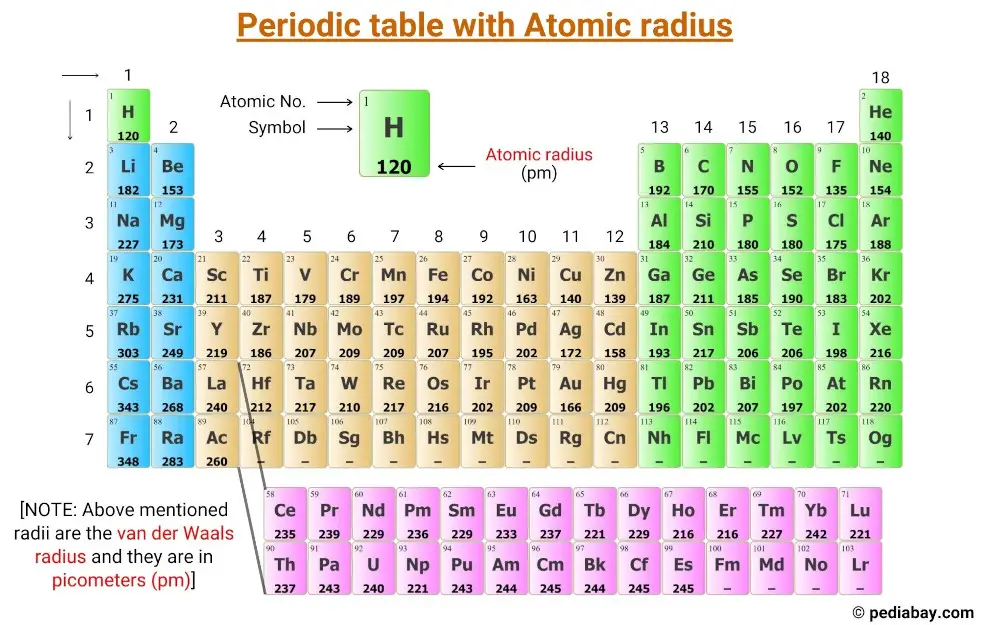
This is a periodic table chart with atomic radius values of elements labeled on it.
The radii mentioned in the above periodic table are the van der Waals radius and they are in picometers (pm).
Well there are few important conceptual things about the atomic radius of the elements that you should know.
So let’s dive right into it.
Table of contents:
What is atomic radius?
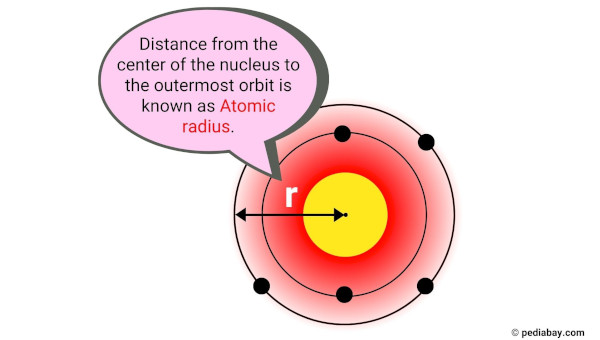
Atomic radius is the distance from the center of an atom’s nucleus to the outermost electron in its electron cloud.
In simpler terms, it is the size of an atom. The atomic radius is usually measured in picometers (pm), which is one trillionth of a meter.
Atoms are extremely small and difficult to visualize, but the concept of atomic radius helps us understand how atoms interact with each other and how they behave in different chemical reactions.
The size of an atom can affect its chemical properties, such as how it bonds with other atoms or molecules.
How is atomic radius measured?
Atomic radius is typically measured using various experimental methods such as X-ray crystallography, electron diffraction, and spectroscopy.
These methods involve studying the behavior of atoms when they interact with certain forms of energy, such as X-rays or electrons.
- In X-ray crystallography, a beam of X-rays is directed at a crystal of the element being studied. The X-rays diffract off the atoms in the crystal, and the resulting diffraction pattern can be used to determine the distance between the atoms and thus their radii.
- In electron diffraction, a beam of electrons is directed at a thin film of the element being studied. As the electrons pass through the film, they scatter off the atoms, creating a diffraction pattern that can be analyzed to determine atomic radius.
- Spectroscopy involves studying the interactions between light and matter. By analyzing the wavelengths of light that are absorbed or emitted by an atom, scientists can determine its atomic radius.
Factors affecting atomic radius
The size of an atom is influenced by various factors, including:
- Nuclear Charge
- Electron Configuration
- Distance of the Valence Electrons from the Nucleus, etc
Nuclear Charge
The number of protons in the nucleus of an atom is known as its atomic number. [1]
As the atomic number increases, the number of protons in the nucleus increases, which results in a greater nuclear charge.
This increased charge attracts electrons more strongly to the nucleus, resulting in a smaller atomic radius.
Electron Configuration
The way electrons are arranged around the nucleus of an atom determines its electron configuration. [2]
The closer the electrons are to the nucleus, the smaller the atomic radius.
Atoms with a larger number of electrons in their outermost energy level (valence shell) will have a larger atomic radius, as the electrons are farther from the nucleus and less strongly attracted to it.
Distance of the Valence Electrons from the Nucleus
The distance of the valence electrons from the nucleus also affects the atomic radius.
As the number of electron shells increases, the valence electrons move farther from the nucleus, resulting in an increase in atomic radius.
It’s important to note that the factors affecting atomic radius are interdependent. For example, while an increase in nuclear charge will result in a smaller atomic radius, an increase in the number of electrons in the outermost energy level (valence shell) will result in a larger atomic radius. Therefore, the overall size of an atom is a result of a balance between these factors.
For more information, you must read the atomic radius trends on the periodic table.
Summary
Atomic radius is the size of an atom, measured in picometers, from the center of the nucleus to the outermost electron in the electron cloud.
It helps us understand how atoms interact and affect their chemical properties. Experimental methods, such as X-ray crystallography, electron diffraction, and spectroscopy, are used to measure atomic radius.
The factors affecting atomic radius are nuclear charge, electron configuration, and distance of the valence electrons from the nucleus. These factors are interdependent and determine the overall size of an atom.
External resources:
- Rana, J. (2022, October 25). Atomic Radius Trend in Periodic Table (Simple Explanation). Knords Learning. https://knordslearning.com/atomic-radius-trend-periodic-table/
- Atomic and ionic radius. (n.d.). Atomic and Ionic Radius. https://www.chemguide.co.uk/atoms/properties/atradius.html
- Atomic radius – Wikipedia. (2021, May 9). Atomic Radius – Wikipedia. https://en.wikipedia.org/wiki/Atomic_radius
- Atomic Radii. (2013, October 2). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Atomic_Radii
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.

