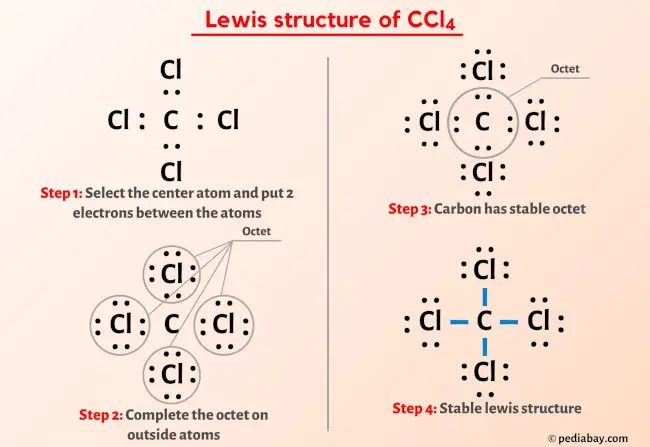
So you have seen the above image by now, right?
Let me explain the above image in short.
CCl4 lewis structure has a Carbon atom (C) at the center which is surrounded by four Chlorine atoms (Cl). There are 4 single bonds between the Carbon atom (C) and each Chlorine atom (Cl). There are 3 lone pairs on all the four Chlorine atoms (Cl).
If you haven’t understood anything from the above image of CCl4 lewis structure, then just stick with me and you will get the detailed step by step explanation on drawing a lewis structure of CCl4.
So let’s move to the steps of drawing the lewis structure of CCl4.
Steps of drawing CCl4 lewis structure
Step 1: Find the total valence electrons in CCl4 molecule
In order to find the total valence electrons in CCl4 molecule, first of all you should know the valence electrons present in carbon atom as well as chlorine atom.
(Valence electrons are the electrons that are present in the outermost orbit of any atom.)
Here, I’ll tell you how you can easily find the valence electrons of carbon as well as chlorine using a periodic table.
Total valence electrons in CCl4 molecule
→ Valence electrons given by carbon atom:
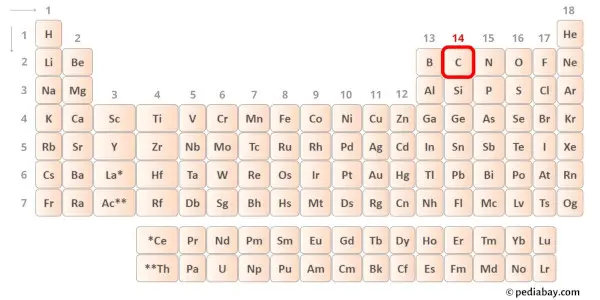
Carbon is group 14 element on the periodic table. [1] Hence the valence electrons present in carbon is 4.
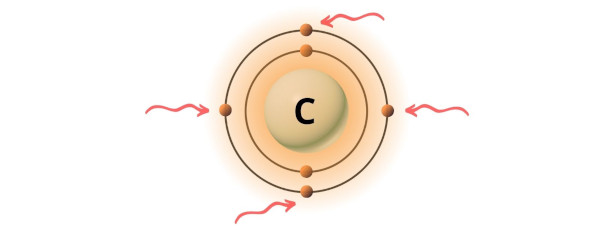
You can see the 4 valence electrons present in the carbon atom as shown in the above image.
→ Valence electrons given by chlorine atom:
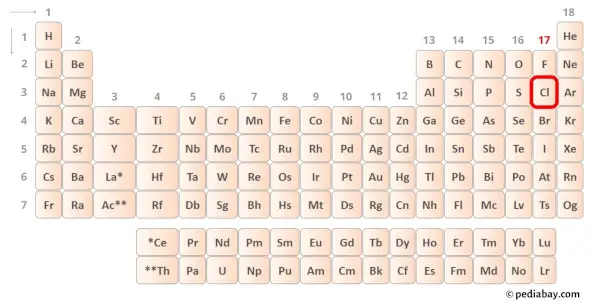
Chlorine is group 17 element on the periodic table. [2] Hence the valence electrons present in chlorine is 7.
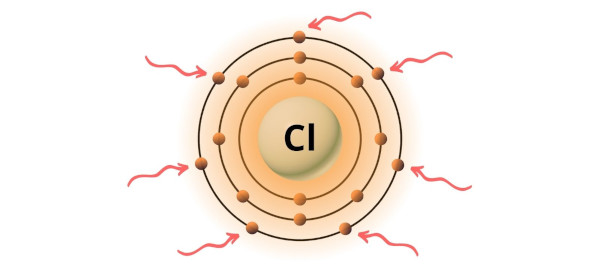
You can see the 7 valence electrons present in the chlorine atom as shown in the above image.
Hence,
Total valence electrons in CCl4 molecule = valence electrons given by 1 carbon atom + valence electrons given by 4 chlorine atoms = 4 + 7(4) = 32.
Step 2: Select the central atom
For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.
Now here the given molecule is CCl4 and it contains carbon atom (C) and chlorine atoms (Cl).
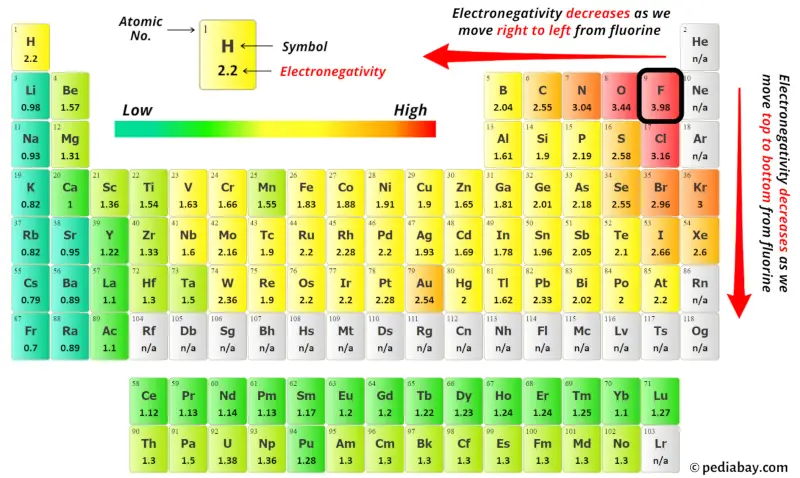
You can see the electronegativity values of carbon atom (C) and chlorine atom (Cl) in the above periodic table.
If we compare the electronegativity values of carbon (C) and chlorine (Cl) then the carbon atom is less electronegative.
So here the carbon atom (C) is the center atom and the chlorine atoms (Cl) are the outside atoms.
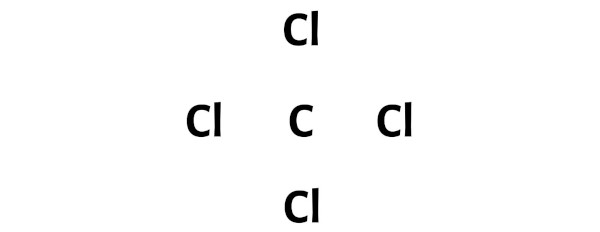
Step 3: Connect each atoms by putting an electron pair between them
Now in the CCl4 molecule, you have to put the electron pairs between the carbon atom (C) and chlorine atoms (Cl).
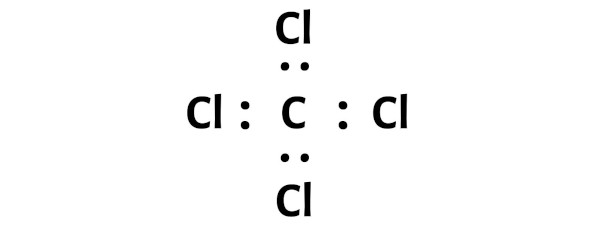
This indicates that the carbon (C) and chlorine (Cl) are chemically bonded with each other in a CCl4 molecule.
Step 4: Make the outer atoms stable
Now in this step, you have to check the stability of the outer atoms.
Here in the sketch of CCl4 molecule, you can see that the outer atoms are chlorine atoms.
These outer chlorine atoms are forming an octet and hence they are stable.
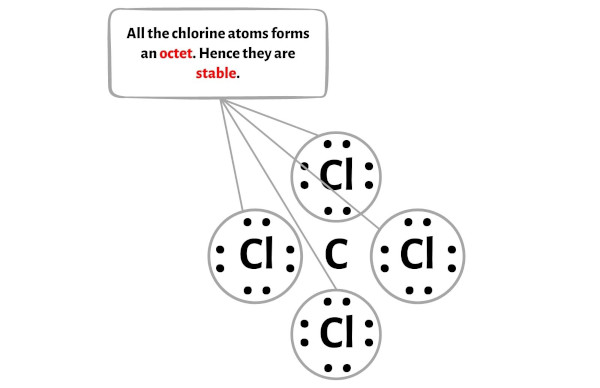
Also, in step 1 we have calculated the total number of valence electrons present in the CCl4 molecule.
The CCl4 molecule has a total 32 valence electrons and all these valence electrons are used in the above sketch of CCl4.
Hence there are no remaining electron pairs to be kept on the central atom.
So now let’s proceed to the next step.
Step 5: Check the octet on the central atom
In this step, you have to check whether the central carbon atom (C) is stable or not.
In order to check the stability of the central carbon (C) atom, we have to check whether it is forming an octet or not.
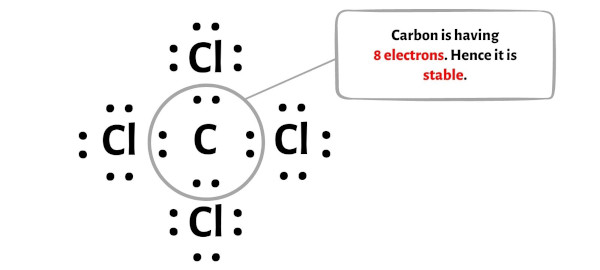
You can see from the above picture that the carbon atom is forming an octet. That means it has 8 electrons.
And hence the central carbon atom is stable.
Now let’s proceed to the final step to check whether the lewis structure of CCl4 is stable or not.
Step 6: Check the stability of lewis structure
Now you have come to the final step in which you have to check the stability of lewis structure of CCl4.
The stability of lewis structure can be checked by using a concept of formal charge.
In short, now you have to find the formal charge on carbon (C) atom as well as chlorine (Cl) atoms present in the CCl4 molecule.
For calculating the formal charge, you have to use the following formula;
Formal charge = Valence electrons – (Bonding electrons)/2 – Nonbonding electrons
You can see the number of bonding electrons and nonbonding electrons for each atom of CCl4 molecule in the image given below.
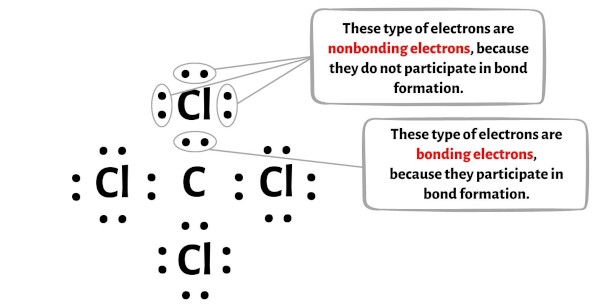
For Carbon (C) atom:
Valence electrons = 4 (because carbon is in group 14)
Bonding electrons = 8
Nonbonding electrons = 0
For Chlorine (Cl) atom:
Valence electron = 7 (because chlorine is in group 17)
Bonding electrons = 2
Nonbonding electrons = 6
| Formal charge | = | Valence electrons | – | (Bonding electrons)/2 | – | Nonbonding electrons | ||
| C | = | 4 | – | 8/2 | – | 0 | = | 0 |
| Cl | = | 7 | – | 2/2 | – | 6 | = | 0 |
From the above calculations of formal charge, you can see that the carbon (C) atom as well as chlorine (Cl) atom has a “zero” formal charge.
This indicates that the above lewis structure of CCl4 is stable and there is no further change in the above structure of CCl4.
In the above lewis dot structure of CCl4, you can also represent each bonding electron pair (:) as a single bond (|). By doing so, you will get the following lewis structure of CCl4.
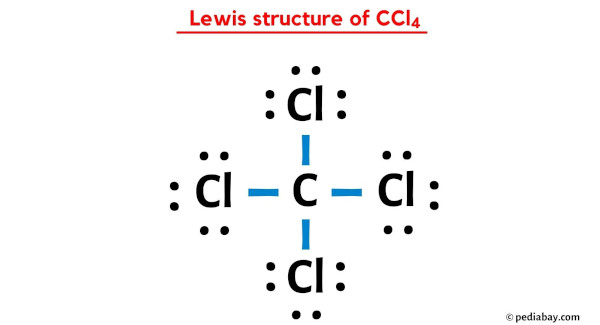
I hope you have completely understood all the above steps.
For more practice and better understanding, you can try other lewis structures listed below.
Try (or at least See) these lewis structures for better understanding:
| CS2 lewis structure | SF6 lewis structure |
| PH3 lewis structure | NO lewis structure |
| N2O lewis structure | CH3OH lewis structure |
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.