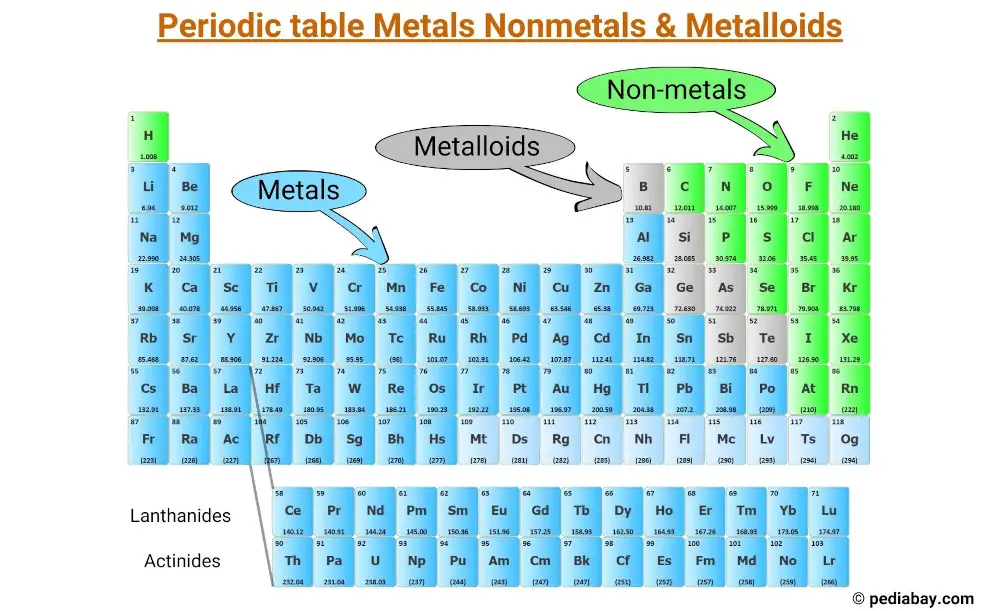
All the elements of the periodic table can be classified into 3 main categories;
- Metals
- Nonmetals and
- Metalloids
Metals are typically shiny, conductive, and ductile, and make up the majority of the elements on the left side of the periodic table.
Nonmetals, on the other hand, tend to be brittle (some are gases) and poor conductors, and are mostly located on the right side of the table.
Metalloids share properties with both metals and nonmetals and are located along the “staircase” that separates the metals and nonmetals.
Let’s explore more about the metals, nonmetals and metalloids of the periodic table.
Metals
Metals make up the majority of elements on the periodic table, and are located on the left side of the “staircase” line that separates metals from nonmetals.
Metals share certain characteristics such as high electrical conductivity, luster, ductility and malleability.
There are five main types of metals:
- Alkali metals, located in group 1 of the periodic table, are soft, highly reactive metals that react violently with water.
- Alkaline earth metals, located in group 2, are also quite reactive, but less so than alkali metals. They are important constituents of minerals in the earth’s crust.
- Transition metals, located in groups 3-11, are some of the most commonly known metals, including iron, gold, and silver. They have high melting points, and often have colorful compounds. [1]
- Lanthanides and actinides, located in the f-block, are known as the “rare earth metals.” They have unique properties such as magnetic and luminescent properties. [2]
- Post transition metals, located outside of the d- and f-blocks, include metals such as aluminum, tin, lead, etc. They are used for a wide range of applications such as construction, transportation, and electronics.
Overall, metals have many important uses in our daily lives, from building structures and machinery to being essential components in modern technology.
Nonmetals
Nonmetals are a group of chemical elements that do not exhibit metallic properties. They are located on the right side of the periodic table, with the exception of hydrogen, which is located at the top left corner.
Nonmetals can be divided into several categories, including:
- Noble gases: These are elements that are stable and inert, meaning they do not easily react with other elements. The noble gases include helium, neon, argon, krypton, xenon, and radon.
- Halogens: These are highly reactive nonmetals that readily form compounds with other elements. The halogens include fluorine, chlorine, bromine, iodine, and astatine.
- Other nonmetals: These include elements such as hydrogen, carbon, nitrogen, oxygen, phosphorus, sulfur, and selenium.
Nonmetals have several physical and chemical properties that distinguish them from metals.
For example, nonmetals tend to be poor conductors of heat and electricity, and they have lower melting and boiling points than metals.
Nonmetals also tend to form negative ions when they react with metals, while metals tend to form positive ions. [3]
Metalloids
Metalloids are elements that share properties with both metals and nonmetals.
They are located along the “staircase” that separates the two categories on the periodic table.
Metalloids includes elements like:
Metalloids tend to have properties that make them useful in technological and industrial applications.
For example, silicon is an important component of semiconductors, while boron is used in the production of strong and lightweight alloys. [4]
Arsenic has been used in various applications including wood preservatives and insecticides, although it is now recognized as a toxic substance. [5]
One key property of metalloids is their ability to conduct electricity under certain conditions.
For example, silicon is a semiconductor, meaning it can be used to control the flow of electricity in electronic devices.
Metalloids are also generally brittle and can fracture under stress, similar to solid nonmetals.
Overall, the unique properties of metalloids make them important elements in a variety of applications, particularly in the field of technology and electronics.
Summary
The periodic table consists of three main categories of elements: metals, nonmetals, and metalloids.
Metals are located on the left side of the periodic table and possess properties such as high electrical conductivity, luster, ductility, and malleability.
Nonmetals, on the other hand, are located on the right side of the periodic table and are generally brittle and poor conductors.
Metalloids share properties with both metals and nonmetals and are located along the “staircase” that separates the two categories.
External resources:
- 6.7: Metalloids. (2016, June 27). Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Introductory_Chemistry_(CK-12)/06%3A_The_Periodic_Table/6.07%3A_Metalloids
- Metal – Wikipedia. (2021, March 25). Metal – Wikipedia. https://en.wikipedia.org/wiki/Metal
- Boudreaux, K. A. (n.d.). The Parts of the Periodic Table. The Parts of the Periodic Table. https://www.angelo.edu/faculty/kboudrea/periodic/physical_metals.htm
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.