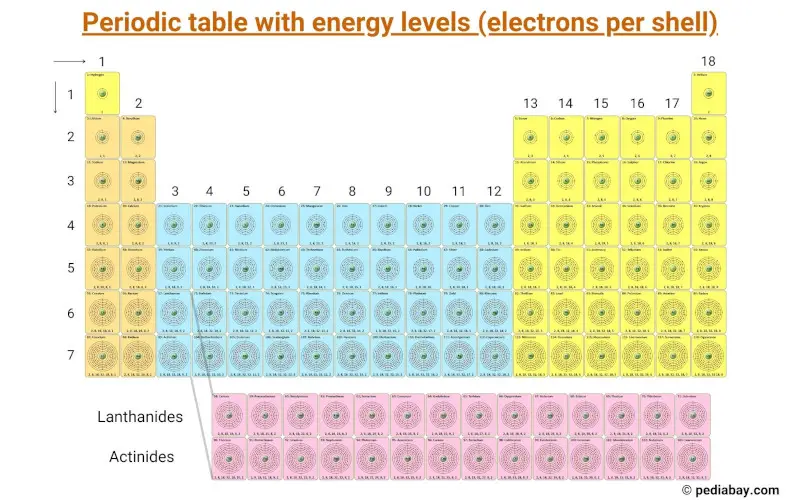
This periodic table contains the Energy levels (electrons per shell) of all the elements.
You can download the HD image from below.
Elements and their electrons per shell arrangement
The electrons arrangement of all the 118 elements are shown in the table below.
| Atomic Number | Element | Electrons per shell |
| 1 | Hydrogen | 1 |
| 2 | Helium | 2 |
| 3 | Lithium | 2, 1 |
| 4 | Beryllium | 2, 2 |
| 5 | Boron | 2, 3 |
| 6 | Carbon | 2, 4 |
| 7 | Nitrogen | 2, 5 |
| 8 | Oxygen | 2, 6 |
| 9 | Fluorine | 2, 7 |
| 10 | Neon | 2, 8 |
| 11 | Sodium | 2, 8 , 1 |
| 12 | Magnesium | 2, 8 , 2 |
| 13 | Aluminum | 2, 8 , 3 |
| 14 | Silicon | 2, 8 , 4 |
| 15 | Phosphorus | 2, 8 , 5 |
| 16 | Sulfur | 2, 8 , 6 |
| 17 | Chlorine | 2, 8 , 7 |
| 18 | Argon | 2, 8 , 8 |
| 19 | Potassium | 2, 8, 8, 1 |
| 20 | Calcium | 2, 8, 8, 2 |
| 21 | Scandium | 2, 8, 9, 2 |
| 22 | Titanium | 2, 8, 10, 2 |
| 23 | Vanadium | 2, 8, 11, 2 |
| 24 | Chromium | 2, 8, 13, 1 |
| 25 | Manganese | 2, 8, 13, 2 |
| 26 | Iron | 2, 8, 14, 2 |
| 27 | Cobalt | 2, 8, 15, 2 |
| 28 | Nickel | 2, 8, 16, 2 |
| 29 | Copper | 2, 8, 18, 1 |
| 30 | Zinc | 2, 8, 18, 2 |
| 31 | Gallium | 2, 8, 18, 3 |
| 32 | Germanium | 2, 8, 18, 4 |
| 33 | Arsenic | 2, 8, 18, 5 |
| 34 | Selenium | 2, 8, 18, 6 |
| 35 | Bromine | 2, 8, 18, 7 |
| 36 | Krypton | 2, 8, 18, 8 |
| 37 | Rubidium | 2, 8, 18, 8, 1 |
| 38 | Strontium | 2, 8, 18, 8, 2 |
| 39 | Yttrium | 2, 8, 18, 9, 2 |
| 40 | Zirconium | 2, 8, 18, 10, 2 |
| 41 | Niobium | 2, 8, 18, 12, 1 |
| 42 | Molybdenum | 2, 8, 18, 13, 1 |
| 43 | Technetium | 2, 8, 18, 13, 2 |
| 44 | Ruthenium | 2, 8, 18, 15, 1 |
| 45 | Rhodium | 2, 8, 18, 16, 1 |
| 46 | Palladium | 2, 8, 18, 18 |
| 47 | Silver | 2, 8, 18, 18, 1 |
| 48 | Cadmium | 2, 8, 18, 18, 2 |
| 49 | Indium | 2, 8, 18, 18, 3 |
| 50 | Tin | 2, 8, 18, 18, 4 |
| 51 | Antimony | 2, 8, 18, 18, 5 |
| 52 | Tellurium | 2, 8, 18, 18, 6 |
| 53 | Iodine | 2, 8, 18, 18, 7 |
| 54 | Xenon | 2, 8, 18, 18, 8 |
| 55 | Caesium | 2, 8, 18, 18, 8, 1 |
| 56 | Barium | 2, 8, 18, 18, 8, 2 |
| 57 | Lanthanum | 2, 8, 18, 18, 9, 2 |
| 58 | Cerium | 2, 8, 18, 19, 9, 2 |
| 59 | Praseodymium | 2, 8, 18, 21, 8, 2 |
| 60 | Neodymium | 2, 8, 18, 22, 8, 2 |
| 61 | Promethium | 2, 8, 18, 23, 8, 2 |
| 62 | Samarium | 2, 8, 18, 24, 8, 2 |
| 63 | Europium | 2, 8, 18, 25, 8, 2 |
| 64 | Gadolinium | 2, 8, 18, 25, 9, 2 |
| 65 | Terbium | 2, 8, 18, 27, 8, 2 |
| 66 | Dysprosium | 2, 8, 18, 28, 8, 2 |
| 67 | Holmium | 2, 8, 18, 29, 8, 2 |
| 68 | Erbium | 2, 8, 18, 30, 8, 2 |
| 69 | Thulium | 2, 8, 18, 31, 8, 2 |
| 70 | Ytterbium | 2, 8, 18, 32, 8, 2 |
| 71 | Lutenium | 2, 8, 18, 32, 9, 2 |
| 72 | Hafnium | 2, 8, 18, 32, 10, 2 |
| 73 | Tantalum | 2, 8, 18, 32, 11, 2 |
| 74 | Tungsten | 2, 8, 18, 32, 12, 2 |
| 75 | Rhenium | 2, 8, 18, 32, 13, 2 |
| 76 | Osmium | 2, 8, 18, 32, 14, 2 |
| 77 | Iridium | 2, 8, 18, 32, 15, 2 |
| 78 | Platinum | 2, 8, 18, 32, 17, 1 |
| 79 | Gold | 2, 8, 18, 32, 18, 1 |
| 80 | Mercury | 2, 8, 18, 32, 18, 2 |
| 81 | Thallium | 2, 8, 18, 32, 18, 3 |
| 82 | Lead | 2, 8, 18, 32, 18, 4 |
| 83 | Bismuth | 2, 8, 18, 32, 18, 5 |
| 84 | Polonium | 2, 8, 18, 32, 18, 6 |
| 85 | Astatine | 2, 8, 18, 32, 18, 7 |
| 86 | Radon | 2, 8, 18, 32, 18, 8 |
| 87 | Francium | 2, 8, 18, 32, 18, 8, 1 |
| 88 | Radium | 2, 8, 18, 32, 18, 8, 2 |
| 89 | Actinium | 2, 8, 18, 32, 18, 9, 2 |
| 90 | Thorium | 2, 8, 18, 32, 18, 10, 2 |
| 91 | Protactinium | 2, 8, 18, 32, 20, 9, 2 |
| 92 | Uranium | 2, 8, 18, 32, 21, 9, 2 |
| 93 | Neptunium | 2, 8, 18, 32, 22, 9, 2 |
| 94 | Plutonium | 2, 8, 18, 32, 24, 8, 2 |
| 95 | Americium | 2, 8, 18, 32, 25, 8, 2 |
| 96 | Curium | 2, 8, 18, 32, 25, 9, 2 |
| 97 | Berkelium | 2, 8, 18, 32, 27, 8, 2 |
| 98 | Californium | 2, 8, 18, 32, 28, 8, 2 |
| 99 | Einsteinium | 2, 8, 18, 32, 29, 8, 2 |
| 100 | Fermium | 2, 8, 18, 32, 30, 8, 2 |
| 101 | Mendelevium | 2, 8, 18, 32, 31, 8, 2 |
| 102 | Nobelium | 2, 8, 18, 32, 32, 8, 2 |
| 103 | Lawrencium | 2, 8, 18, 32, 32, 8, 3 |
| 104 | Rutherfordium | 2, 8, 18, 32, 32, 10, 2 |
| 105 | Dubnium | 2, 8, 18, 32, 32, 11, 2 |
| 106 | Seaborgium | 2, 8, 18, 32, 32, 12, 2 |
| 107 | Bohrium | 2, 8, 18, 32, 32, 13, 2 |
| 108 | Hasnium | 2, 8, 18, 32, 32, 14, 2 |
| 109 | Meitnerium | 2, 8, 18, 32, 32, 15, 2 |
| 110 | Darmstadtium | 2, 8, 18, 32, 32, 17, 1 |
| 111 | Roentgenium | 2, 8, 18, 32, 32, 17, 2 |
| 112 | Copernicium | 2, 8, 18, 32, 32, 18, 2 |
| 113 | Nihonium | 2, 8, 18, 32, 32, 18, 3 |
| 114 | Flerovium | 2, 8, 18, 32, 32, 18, 4 |
| 115 | Moscovium | 2, 8, 18, 32, 32, 18, 5 |
| 116 | Livermorium | 2, 8, 18, 32, 32, 18, 6 |
| 117 | Tennessine | 2, 8, 18, 32, 32, 18, 7 |
| 118 | Oganesson | 2, 8, 18, 32, 32, 18, 8 |
External resources:
- Electron shell – Wikipedia. (n.d.). Electron Shell – Wikipedia. https://en.wikipedia.org/wiki/Electron_shell
- 2.5: Arrangement of Electron (Shell Model). (2014, July 3). Chemistry LibreTexts. https://chem.libretexts.org/Courses/Sacramento_City_College/SCC%3A_Chem_309_-_General_Organic_and_Biochemistry_(Bennett)/Text/02._Atomic_Structure/2.5%3A_Arrangement_of_Electron_(Shell_Model)
About author
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.

