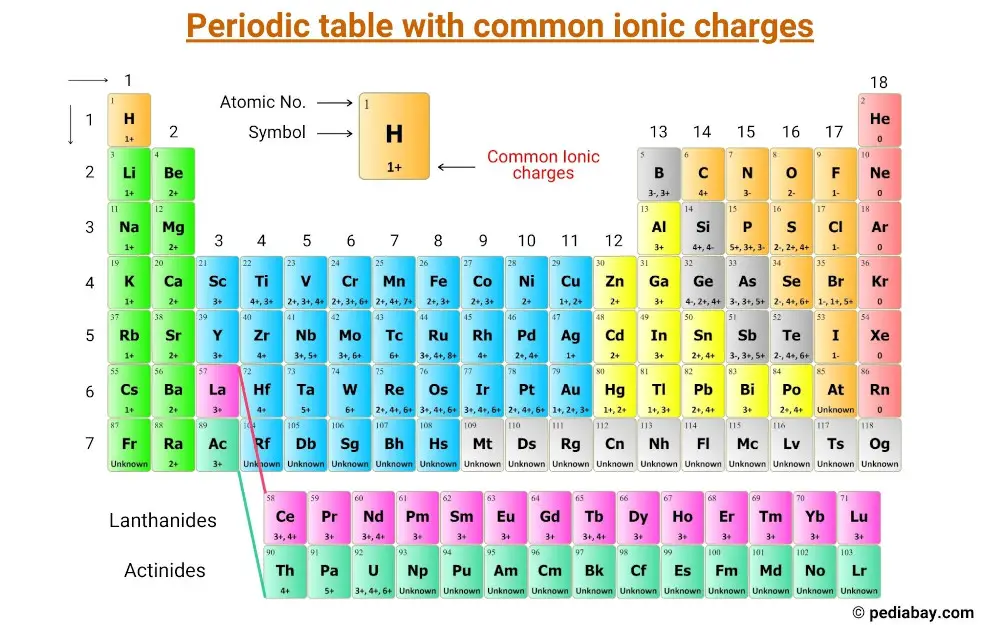
This is a periodic table with common ionic charges of elements labeled on it.
You can also download the HD image from here;
List of elements with their common ionic charges
The common ionic charges of the elements are shown in the table below.
| Atomic number of element | Ion | Common charge/s |
| 1 | Hydrogen ion | 1+ |
| 2 | Helium ion | 0 |
| 3 | Lithium ion | 1+ |
| 4 | Beryllium ion | 2+ |
| 5 | Boron ion | 3-, 3+ |
| 6 | Carbon ion | 4+ |
| 7 | Nitrogen ion | 3- |
| 8 | Oxygen ion | 2- |
| 9 | Fluorine ion | 1- |
| 10 | Neon ion | 0 |
| 11 | Sodium ion | 1+ |
| 12 | Magnesium ion | 2+ |
| 13 | Aluminum ion | 3+ |
| 14 | Silicon ion | 4+, 4- |
| 15 | Phosphorus ion | 5+, 3+, 3- |
| 16 | Sulfur ions | 2-, 2+, 4+, 6+ |
| 17 | Chlorine ion | 1- |
| 18 | Argon ion | 0 |
| 19 | Potassium ion | 1+ |
| 20 | Calcium ion | 2+ |
| 21 | Scandium ion | 3+ |
| 22 | Titanium ion | 4+, 3+ |
| 23 | Vanadium ion | 2+, 3+, 4+, 5+ |
| 24 | Chromium ion | 2+, 3+,6+ |
| 25 | Manganese ion | 2+, 4+, 7+ |
| 26 | Iron ion | 2+, 3+ |
| 27 | Cobalt ion | 2+, 3+ |
| 28 | Nickel ion | 2+ |
| 29 | Copper ion | 1+, 2+ |
| 30 | Zinc ion | 2+ |
| 31 | Gallium ion | 3+ |
| 32 | Germanium ion | 4-, 2+, 4+ |
| 33 | Arsenic ions | 3-, 3+, 5+ |
| 34 | Selenium ion | 2-, 4+, 6+ |
| 35 | Bromine ion | 1-, 1+, 5+ |
| 36 | Krypton ion | 0 |
| 37 | Rubidium ion | 1+ |
| 38 | Strontium ion | 2+ |
| 39 | Yttrium ion | 3+ |
| 40 | Zirconium ion | 4+ |
| 41 | Niobium ion | 3+, 5+ |
| 42 | Molybdenum ion | 3+, 6+ |
| 43 | Technetium ion | 6+ |
| 44 | Ruthenium ion | 3+, 4+, 8+ |
| 45 | Rhodium ion | 4+ |
| 46 | Palladium ion | 2+, 4+ |
| 47 | Silver ion | 1+ |
| 48 | Cadmium ion | 2+ |
| 49 | Indium ion | 3+ |
| 50 | Tin ion | 2+, 4+ |
| 51 | Antimony ion | 3-, 3+, 5+ |
| 52 | Tellurium ion | 2-, 4+, 6+ |
| 53 | Iodine ion | 1- |
| 54 | Xenon ion | 0 |
| 55 | Cesium ion | 1+ |
| 56 | Barium ion | 2+ |
| 57 | Lanthanum ion | 3+ |
| 58 | Cerium ion | 3+, 4+ |
| 59 | Praseodymium ion | 3+ |
| 60 | Neodymium ion | 3+, 4+ |
| 61 | Promethium ion | 3+ |
| 62 | Samarium ion | 3+ |
| 63 | Europium ion | 3+ |
| 64 | Gadolinium ion | 3+ |
| 65 | Terbium ion | 3+, 4+ |
| 66 | Dysprosium ion | 3+ |
| 67 | Holmium ion | 3+ |
| 68 | Erbium ion | 3+ |
| 69 | Thulium ion | 3+ |
| 70 | Ytterbium ion | 3+ |
| 71 | Lutetium ion | 3+ |
| 72 | Hafnium ion | 4+ |
| 73 | Tantalum ion | 5+ |
| 74 | Tungsten ion | 6+ |
| 75 | Rhenium ion | 2+, 4+, 6+, 7+ |
| 76 | Osmium ion | 3+, 4+, 6+, 8+ |
| 77 | Iridium ion | 3+, 4+, 6+ |
| 78 | Platinum ion | 2+, 4+, 6+ |
| 79 | Gold ion | 1+, 2+, 3+ |
| 80 | Mercury ion | 1+, 2+ |
| 81 | Thallium ion | 1+, 3+ |
| 82 | Lead ion | 2+, 4+ |
| 83 | Bismuth ion | 3+ |
| 84 | Polonium ion | 2+, 4+ |
| 85 | Astatine ion | Unknown |
| 86 | Radon ion | 0 |
| 87 | Francium ion | Unknown |
| 88 | Radium ion | 2+ |
| 89 | Actinium ion | 3+ |
| 90 | Thorium ion | 4+ |
| 91 | Protactinium ion | 5+ |
| 92 | Radon ion | 3+, 4+, 6+ |
External resources:
- Rana, J. (2022, October 26). Ionic Charges of All Elements. Knords Learning. https://knordslearning.com/ionic-charges-of-elements-periodic-table/
- A. (2021, February 26). Ionic Charges of All Elements (List + Images inside). Periodic Table Guide. https://periodictableguide.com/ionic-charges-of-all-elements-list/
About author
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.