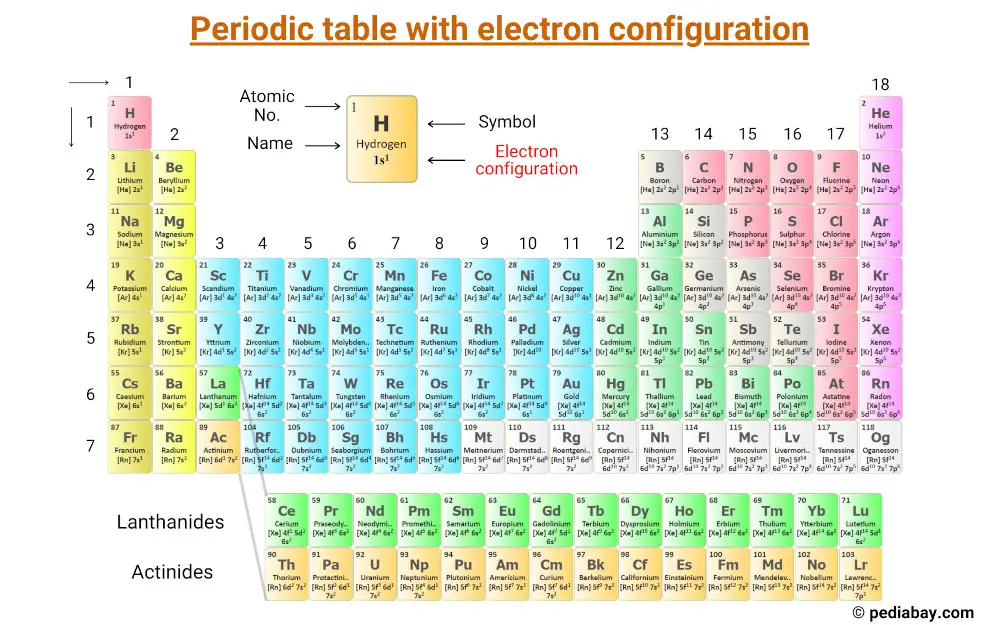
This is a periodic table with electron configuration of elements labeled on it.
You can also download the HD image from here;
Let’s explore more about the electron configurations of the elements;
Table of contents:
What is an electronic configuration?
Electronic configuration refers to the arrangement of electrons in an atom.
Electrons are tiny negatively charged particles that orbit the positively charged nucleus of an atom.
The electron configuration of an atom determines its chemical and physical properties, such as its reactivity, conductivity, and melting point.
The arrangement of electrons in an atom is determined by its energy levels (which are also called shells), and the specific subshells within those shells.
Electron configuration is typically represented using a notation that indicates the number of electrons in each shell and subshell, and it follows certain rules based on the principles of quantum mechanics.
What are Shells (n) and Subshells (s, p, d, f)?
Shell: The shell is the orbit in which the electrons revolve around the nucleus of an atom.
Each shell is assigned a number (n), starting from one for the first shell and increasing with each additional shell.
The first shell can only hold a maximum of 2 electrons, while the second shell can hold up to 8 electrons, and subsequent shells can hold even more.
Subshell (or Orbitals): Within each shell, there are different subshells, which are labeled using letters such as s, p, d, and f.
The s subshell can hold a maximum of 2 electrons, the p subshell can hold up to 6 electrons, the d subshell can hold up to 10 electrons, and the f subshell can hold up to 14 electrons. [1]
The shells and subshells of an atom determine its electron configuration, which in turn determines its chemical and physical properties.
Electron configuration of elements (List)
Here is the list of electron configurations of the first 20 elements of periodic table.
| Atomic number | Element | Electron configuration |
| 1 | Hydrogen (H) | 1s1 |
| 2 | Helium (He) | 1s2 |
| 3 | Lithium (Li) | [He] 2s1 |
| 4 | Beryllium (Be) | [He] 2s2 |
| 5 | Boron (B) | [He] 2s2 2p1 |
| 6 | Carbon (C) | [He] 2s2 2p2 |
| 7 | Nitrogen (N) | [He] 2s2 2p3 |
| 8 | Oxygen (O) | [He] 2s2 2p4 |
| 9 | Fluorine (F) | [He] 2s2 2p5 |
| 10 | Neon (Ne) | [He] 2s2 2p6 |
| 11 | Sodium (Na) | [Ne] 3s1 |
| 12 | Magnesium (Mg) | [Ne] 3s2 |
| 13 | Aluminum (Al) | [Ne] 3s2 3p1 |
| 14 | Silicon (Si) | [Ne] 3s2 3p2 |
| 15 | Phosphorus (P) | [Ne] 3s2 3p3 |
| 16 | Sulfur (S) | [Ne] 3s2 3p4 |
| 17 | Chlorine (Cl) | [Ne] 3s2 3p5 |
| 18 | Argon (Ar) | [Ne] 3s2 3p6 |
| 19 | Potassium (K) | [Ar] 4s1 |
| 20 | Calcium (Ca) | [Ar] 4s2 |
External resources:
- 2.4 Electron Configurations. (2016, May 13). Chemistry LibreTexts. https://chem.libretexts.org/Courses/Valley_City_State_University/Chem_115/Chapter_2%3A_Atomic_Structure/2.4_Electron_Configurations
- Electron configuration – Wikipedia. (2018, June 12). Electron Configuration – Wikipedia. https://en.wikipedia.org/wiki/Electron_configuration
Jay is an educator and has helped more than 100,000 students in their studies by providing simple and easy explanations on different science-related topics. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations.
Read more about our Editorial process.